Radiofrequency ablation and breast cancer: a review
Introduction
Globally, breast cancer is the most frequently diagnosed and the leading cause of cancer death in women. In 2013, the World Health Organization’s International Agency for Research on Cancer noted a sharp rise in breast cancer worldwide, an increase in more than 20% since 2008. The economic and social burden is great, as breast cancer represents one in four of all cancers in women (1,2). The current standard of treatment for breast cancer is surgery. Surgical considerations include the staging of the cancer with the Tumor, Node, Metastases system and the size of the tumor relative to the remaining breast tissue. For patients with non-metastatic breast cancer, stratification is generally to either early stage, clinical stage I, IIA, or IIB (T2N1) or locally advanced breast cancer, clinical stage IIB (T3N0) to IIIC (3). Options for surgery range from lumpectomy, simple resection of the tumor and margin of healthy breast tissue, to total mastectomy with sentinel node biopsy for axillary staging. Although decision-making is prioritized to obtain best oncological outcomes, factors such as breast conservation and cosmetic outcomes are also important considerations with breast cancer treatment.
Surgical resection has been the standard of treatment of primary solid tumors localized to organs such as the lung, colon, and breast. New research in radiofrequency ablative therapy has brought light to alterative noninvasive methods (4). Nonsurgical options have a number of benefits, including decreased risk of complications from anesthesia, improved cosmesis, and shortened recovery time. Many patients have co-morbidities and poor functional status, which increase risk of post-surgical complications including bleeding, stroke, and/or death (5). In these cases, a nonsurgical option may decrease the morbidity and mortality of patients. Improved cosmesis, such as breast conservation and minimal scarring, is another potential benefit from less invasive breast cancer treatment. Unlike other solid tumors, breast cancer is unique in the importance of decreasing the deformity of the surgical procedure. In a 2000 retrospective study by Al-Ghazal et al., it was concluded that patient satisfaction with cosmetic outcomes after breast cancer therapy was critical to psychological well-being and quality of life (6).
Radiofrequency ablation (RFA) is a widely studied minimally invasive technique. It is used as treatment and palliation in a number of primary and secondary solid tumors including hepatocellular carcinoma, non-small cell lung cancer, and renal neoplasms (7). RFA utilizes a radiofrequency electrode and image guidance, usually CT or ultrasound, to heat and coagulate targeted tissue (8). The thermal energy is localized to achieve necrosis of only malignant tissue with minimal destruction of the surrounding healthy cells. RFA use in breast cancer is a developing area of research, and particularly exciting due to the recent trend toward less invasive breast cancer treatments. There have been a number of published studies over the last decade which explore the feasibility of minimally invasive techniques in breast cancer treatment. In this review paper, we will discuss the most recent data on radiofrequency ablation and examine the current methods, outcomes, complications, and limitations of RFA in breast cancer therapy.
Results
English-language papers on RFA use in breast cancer are summarized in Table 1. Most of the articles are small feasibility studies involving invasive breast tumors, with the N representing the number of tumors. The majority of studies were from single institution populations, however, one multicenter study was included in this review (21). A few studies included are follow-up papers from previous studies (25,29,31). Due to the technical limitations of RFA, all tumors were selected on basis of size. Although studies had various upper limits, the average tumor size was relatively comparable at 1-2 cm. Some studies reported only tumor staging; therefore no average tumor size was obtained (9,19). Since RFA techniques are both operator and instrument dependent, we included the type of RFA electrode utilized. The two most common electrodes used were Cool-Tip by Covidien and Starburst by Angiodynamics. In this review, complete ablation is defined as the absence of viable tumor cells in the resected specimen as verified by immunohistochemistry, tissue biopsy, and/or imaging. The table shows both the number and percentage of complete ablation reported by each study. Surgical resection usually occurred after RFA was applied in vivo. In three cases, surgical resection occurred first, and RFA therapy was later applied in vitro (15,33,35). In a number of cases, definitive surgical resection was not performed due to age of patient or co-morbidities that did not allow for mass excision (16,17,19,20,26,28,32,34). In these cases, tissue may only be obtained via ultrasound guided core biopsy or mammotome biopsy. Skin burn and mass formation at the electrode site were the major RFA complications reported by the studies, although this occurred in only a small number of patients.
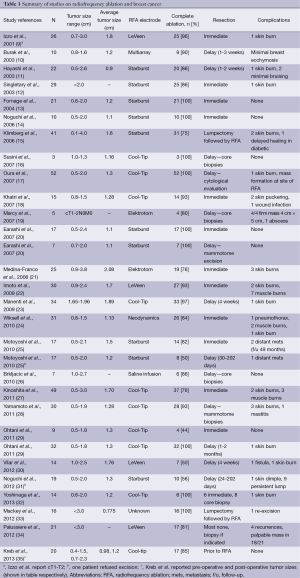
Full table
Discussion
Patient selection criteria
RFA utilizes local thermal energy to induce coagulative necrosis, which limits the size of tumors eligible for ablation. The participants were therefore highly selected based on patient factors, features on initial biopsy, and imaging. Selective criteria between studies differed greatly. For instance, patients with prior neoadjuvant chemotherapy were excluded from some studies; while in others, it was the near majority of the patient population (34). Others made amendments to their criteria to include patients receiving neoadjuvant chemotherapy later in the study (13). In addition, many studies excluded patients on the basis of features found on biopsy such as signs of ductal carcinoma in situ and lobular type carcinoma. Some studies also excluded patients on the basis of imaging findings such as masses with unclear borders, extensive microcalcifications, and tumors within 1 cm of the skin (10,29). Since thermal ablation is known to change the characteristics of tumor marker expression, estrogen, progesterone, and HER2 receptor status, grade, histology, and need for adjuvant chemotherapy had to be known prior to RFA therapy (11,13,18,22). Studies with planned definitive resection excluded patients with medical contraindications to surgery, such as sensitivity to anesthesia and coagulopathy. However, there were also a number of studies where the population of interest was specifically elderly patients who refused surgery and/or had co-morbid conditions that precluded them from surgical interventions (16,19,34). Due to strict and varying nature of the patients selected for the studies described in this review, the application of RFA therapy may not be feasible in a significant portion of breast cancer patients. Nevertheless as the median sizes of the breast tumor are decreasing due to population screening increasing number of patients may be eligible.
RFA devices
Studies in this review utilized the RFA electrode listed in Table 1. These RFA instruments were originally developed for non-breast cancer solid tumors and vary in both the shape and size of needle and array.
The Covidien Cool Tip was developed for soft tissue tumors. It involves a system of straight needles which when used in combination can ablate an area of up to 6.7 cm × 6.5 cm. It uses a 20.5-gauge needle with lengths varying from 10-25 cm. The cool tip system uses water during the process to reduce impedance of the tissues during ablation (36).
Integra makes the Elektrotom HiTT and like the Cool tip has a saline infused electrode and developed for liver tumor ablation. This comes in a variety of straight needle lengths and electrodes. It is compatible with all imaging modalities (37).
The LeVeen by Boston Scientific RFA device was designed for the ablation of liver tumors with a straight needle with umbrella shaped array. Lengths range from 12-25 cm with array diameters from 2-5 cm (38).
Starburst by Angiodynamics was developed for soft tissue, liver and bone. It is able to curve up to 90 degrees with an umbrella deployment of electrodes, is MRI compatible with a retractable/deployable needle and can ablate up to a 5 cm diameter from the tip (39).
Radioablation has shown good success in homogenous tissues, such as the liver (40). Since the process of RFA coagulates tumors at different rates depending on the amount of glandular tissue, skin burns are common complications. Breast cancer therapy may benefit from the development of an instrument that can adapt to the heterogeneous nature of breast tissue. Another limitation of RFA therapy is the size of the lesion treated is limited by electrode. Currently, there are no RFA electrodes FDA approved specifically for breast. The general approval is for soft tissue ablation which includes breast.
Ablation success
The method of assessing complete ablation is not mentioned in Table 1. The studies used various methods including immunohistochemistry staining, cytological evaluation, core biopsies, mammotome biopsies, and fine needle aspiration. Most commonly, hematoxylin and eosin (H&E) staining was used to determine the margins of the tumor and nicotinamide adenine dinucleotide (NADH) diaphorase stains were used to detect any viable tumor cells present in the surgical resection. Some studies used cytokeratin 8/18, which detects tumor necrosis seen with successful RFA treatment (10). Using these methods, the majority of the studies showed promising rates of complete ablation. Although histopathology seems to be an adequate way of determining level of ablation with feasibility studies, evaluation requires some form of tissue sampling or excision. While surgical excision and immunohistochemistry will confirm adequate treatment, core biopsies cannot check for tumor-free margins. Therefore, these methods may not be practical for use if the goal is a noninvasive therapy. In these situations, advanced imaging would be necessary for close surveillance and this can include contrast enhanced breast MRI for example. Furthermore the oncology community will need to become comfortable with radiologic assessment of complete tumor destruction (verified by well designed clinical trials) and decision for adjuvant therapy can be made from the pre-therapy core biopsy.
Imaging
The feasibility of RFA use in the treatment of breast cancer will rely largely on the continued advancement of imaging. For one, imaging is critical for the localization of the tumor itself. Many studies compared ultrasound estimates of breast tumors pre-RFA and noted significant variations with respective MRI comparison (10,11,23,30). Secondly, image-guidance is required while performing RFA in vivo to ensure correct placement of the electrode and response of the tumor during the procedure. Incomplete ablation has been linked to incorrect placement of the RFA electrode during ablation, which should be at the center of the tumor (41). Therefore, the effectiveness of RFA treatment is directly correlated to the use of imaging. Radiofrequency ablation utilizes high temperatures, which can lead to complications such as skin burns. Nahirnyak et al. have found a strong link between Doppler ultrasound signal changes and tissue boiling (42). This study suggests that use of Doppler ultrasound following ablation may help to prevent complications due to RFA temperatures. Additionally, imaging is required to assess the tissue response to RFA therapy and the oncologic outcomes of the treatment in long-term follow-up. In the many studies, complete ablation was evaluated with the use of imaging and histopathology. In Palussiere et al. no definitive surgical resection was performed, therefore imaging was the only method of assessing for the successful ablation of lesions (34). Continued advances in imaging will be critical to allow accurate assessment of the ablation and for subsequent follow-up to detect recurrence.
Cosmesis
Breast conserving therapy, breast conserving surgery with adjuvant chemotherapy, has been found to be equivalent to mastectomy, but with the advantage of minimizing deformity to the breast. However, breast conserving therapy still requires negative margins and therefore, may not produce good cosmetic results in patients with less breast tissue. A major benefit of RFA utilization in breast cancer treatment is the potential for improved cosmesis with patients who have early-staged small breast cancers. A few studies evaluated the cosmetic outcomes and patient satisfaction with the procedure (17,23,31) Oura et al. reported cosmetic outcomes as excellent, good and fair, with 43/52 patients reporting excellent cosmesis (17). The major factor affecting the cosmetic results in this study was the presence of mass formation at the RFA site secondary to fat necrosis. Manenti et al. evaluated cosmesis by two surgeons not involved patient treatment, whom evaluated skin texture and pigmentation and rated as excellent, good, acceptable, and poor (23). Overall cosmesis in this study was promising, with 28/34 patients assessed as excellent. Cosmesis may not be accurately reflected in the majority of these studies because surgical excision was used to both confirm the effectiveness of the RFA therapy, as well as ensure the current standard of care was performed. Further study is warranted to evaluate patient satisfaction and cosmetic outcomes in patients without definitive surgical resection.
Complications/safety
Radiofrequency ablation in other cancer therapy has been shown to have a relatively low associated risk, with morbidity between 2-10% and mortality between 0.3-0.8% (40,43). In the above studies, complications associated with RFA included skin burns, muscle burns, ecchymosis, skin puckering and mass formation secondary to fat necrosis at the RFA site. An isolated case of wound infection, mastitis, and pneumothorax were reported in three different studies (18,24,28). Distant metastases were also documented in one study (25). Overall, the safety profile of RFA therapy appears very good, with many of the common complications involving superficial injury or skin changes. In addition, many authors have developed protocols with RFA electrode use to minimize the complications noted above. Oura et al. found fewer skin burns with injection of 5% glucose, which both enlarged the distance between the tumor and skin, as well as interrupting radiofrequency to affected skin (17). Ice packs were applied to the skin in studies when the mass was more superficial to prevent burns (34). Although it appears that many of the common complications are benign skin changes undoubtedly affect the cosmesis and patient satisfaction with the procedure. In addition, a mass formation may increase anxiety of cancer recurrence for the patient. Adequate counseling should be provided prior to RFA treatment to warn patients about these potential results. Future studies may want to optimize thermoablation methods using previous protocols which haven shown success at minimizing these outcomes. As stated previously, there is no current consensus on RFA device or technique in breast cancer masses.
The main safety issue with RFA treatment involves outcomes of breast cancer recurrence and survival rates. As of now, follow up has been largely limited. The longest follow up study is with Noguchi with a mean of 60 months, which found no in-breast recurrence, but one axillary lymph node and hepatic metastasis (31). In terms of oncologic outcome, there is not enough data to conclude if RFA is a safe alternative to surgery at this time.
Conclusions
Radiofrequency ablation is an emerging minimally invasive therapy in small, localized breast cancer. As of now, RFA is FDA approved for use in soft tissue tumors, including breast cancer. With the potential of improved cosmesis and excellent safety profile, RFA may come to replace or compete with current surgical resection if the oncologic outcomes are comparable. Currently, no clinical trials have been conducted to directly compare RFA to the current standard of surgical resection. Comparison studies have been performed in a number of other solid tumors with encouraging results. For instance, Chadwick et al. compared RFA and surgical resection in the treatment of Barrett’s esophagus and found to be the two modalities to be equally effective (44). The limitation with Chadwick’s study, and others of its kind, is that mean follow up was not sufficient to make conclusive statements in long-term oncologic outcomes. In order to assess the role of RFA in breast cancer therapy, imaging modalities will have to advance to detect tumor resolution and monitor for recurrence. Future studies should also document hospital time, post-procedural pain, other complications associated with RFA versus surgical resection. In addition, patient satisfaction with cosmesis and level of anxiety with this novel intervention should also be reported. Ultimately, RFA will need several studies to evaluate for oncologic outcomes these studies requiring a large patient population and long interval follow up to determine disease progression.
There are currently no RFA devices specific for the treatment of breast cancers, nor consensus on how they should be used. With this evolving treatment modality, the next step would involve more studies are warranted to determine which devices work best in the area of breast versus more dense tissues such as liver and bone.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013;132:1133-45. [PubMed]
- Ferlay J, Soerjomataram I, Ervik M, et al. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France, 2013.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Mirza AN, Fornage BD, Sneige N, et al. Radiofrequency ablation of solid tumors. Cancer J 2001;7:95-102. [PubMed]
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol 2007;50:1707-32. [PubMed]
- Al-Ghazal SK, Fallowfield L, Blamey RW. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer 2000;36:1938-43. [PubMed]
- Shah DR, Green S, Elliot A, et al. Current oncologic applications of radiofrequency ablation therapies. World J Gastrointest Oncol 2013;5:71-80. [PubMed]
- Gazelle GS, Goldberg SN, Solbiati L, et al. Tumor ablation with radio-frequency energy. Radiology 2000;217:633-46. [PubMed]
- Izzo F, Thomas R, Delrio P, et al. Radiofrequency ablation in patients with primary breast carcinoma: a pilot study in 26 patients. Cancer 2001;92:2036-44. [PubMed]
- Burak WE Jr, Agnese DM, Povoski SP, et al. Radiofrequency ablation of invasive breast carcinoma followed by delayed surgical excision. Cancer 2003;98:1369-76. [PubMed]
- Hayashi AH, Silver SF, van der Westhuizen NG, et al. Treatment of invasive breast carcinoma with ultrasound-guided radiofrequency ablation. Am J Surg 2003;185:429-35. [PubMed]
- Singletary ES. Feasibility of radiofrequency ablation for primary breast cancer. Breast Cancer 2003;10:4-9. [PubMed]
- Fornage BD, Sneige N, Ross MI, et al. Small (< or = 2-cm) breast cancer treated with US-guided radiofrequency ablation: feasibility study. Radiology 2004;231:215-24. [PubMed]
- Noguchi M, Earashi M, Fujii H, et al. Radiofrequency ablation of small breast cancer followed by surgical resection. J Surg Oncol 2006;93:120-8. [PubMed]
- Klimberg VS, Kepple J, Shafirstein G, et al. eRFA: excision followed by RFA-a new technique to improve local control in breast cancer. Ann Surg Oncol 2006;13:1422-33. [PubMed]
- Susini T, Nori J, Olivieri S, et al. Radiofrequency ablation for minimally invasive treatment of breast carcinoma. A pilot study in elderly inoperable patients. Gynecol Oncol 2007;104:304-10. [PubMed]
- Oura S, Tamaki T, Hirai I, et al. Radiofrequency ablation therapy in patients with breast cancers two centimeters or less in size. Breast Cancer 2007;14:48-54. [PubMed]
- Khatri VP, McGahan JP, Ramsamooj R, et al. A phase II trial of image-guided radiofrequency ablation of small invasive breast carcinomas: use of saline-cooled tip electrode. Ann Surg Oncol 2007;14:1644-52. [PubMed]
- Marcy PY, Magne N, Castadot P, et al. Ultrasound-guided percutaneous radiofrequency ablation in elderly breast cancer patients: preliminary institutional experience. Br J Radiol 2007;80:267-73. [PubMed]
- Earashi M, Noguchi M, Motoyoshi A, et al. Radiofrequency ablation therapy for small breast cancer followed by immediate surgical resection or delayed mammotome excision. Breast Cancer 2007;14:39-47. [PubMed]
- Medina-Franco H, Soto-Germes S, Ulloa-Gomez JL, et al. Radiofrequency ablation of invasive breast carcinomas: a phase II trial. Ann Surg Oncol 2008;15:1689-95. [PubMed]
- Imoto S, Wada N, Sakemura N, et al. Feasibility study on radiofrequency ablation followed by partial mastectomy for stage I breast cancer patients. Breast 2009;18:130-4. [PubMed]
- Manenti G, Bolacchi F, Perretta T, et al. Small breast cancers: in vivo percutaneous US-guided radiofrequency ablation with dedicated cool-tip radiofrequency system. Radiology 2009;251:339-46. [PubMed]
- Wiksell H, Lofgren L, Schassburger KU, et al. Feasibility study on the treatment of small breast carcinoma using percutaneous US-guided preferential radiofrequency ablation (PRFA). Breast 2010;19:219-25. [PubMed]
- Motoyoshi A, Noguchi M, Earashi M, et al. Histopathological and immunohistochemical evaluations of breast cancer treated with radiofrequency ablation. J Surg Oncol 2010;102:385-91. [PubMed]
- Brkljacic B, Cikara I, Ivanac G, et al. Ultrasound-guided bipolar radiofrequency ablation of breast cancer in inoperable patients: a pilot study. Ultraschall in der Medizin 2010;31:156-62. [PubMed]
- Kinoshita T, Iwamoto E, Tsuda H, et al. Radiofrequency ablation as local therapy for early breast carcinomas. Breast Cancer 2011;18:10-7. [PubMed]
- Yamamoto N, Fujimoto H, Nakamura R, et al. Pilot study of radiofrequency ablation therapy without surgical excision for T1 breast cancer: evaluation with MRI and vacuum-assisted core needle biopsy and safety management. Breast Cancer 2011;18:3-9. [PubMed]
- Ohtani S, Kochi M, Ito M, et al. Radiofrequency ablation of early breast cancer followed by delayed surgical resection--a promising alternative to breast-conserving surgery. Breast 2011;20:431-6. [PubMed]
- Vilar VS, Goldman SM, Ricci MD, et al. Analysis by MRI of residual tumor after radiofrequency ablation for early stage breast cancer. AJR Am J Roentgenol 2012;198:W285-91. [PubMed]
- Noguchi M, Motoyoshi A, Earashi M, et al. Long-term outcome of breast cancer patients treated with radiofrequency ablation. Eur J Surg Oncol 2012;38:1036-42. [PubMed]
- Yoshinaga Y, Enomoto Y, Fujimitsu R, et al. Image and pathological changes after radiofrequency ablation of invasive breast cancer: a pilot study of nonsurgical therapy of early breast cancer. World J Surg 2013;37:356-63. [PubMed]
- Mackey A, Feldman S, Vaz A, et al. Radiofrequency ablation after breast lumpectomy added to extend intraoperative margins in the treatment of breast cancer (ABLATE): a single-institution experience. Ann Surg Oncol 2012;19:2618-9. [PubMed]
- Palussière J, Henriques C, Mauriac L, et al. Radiofrequency ablation as a substitute for surgery in elderly patients with nonresected breast cancer: pilot study with long-term outcomes. Radiology 2012;264:597-605. [PubMed]
- Kreb DL, Looij BG, Ernst MF, et al. Ultrasound-guided radiofrequency ablation of early breast cancer in a resection specimen: lessons for further research. Breast 2013;22:543-7. [PubMed]
- Covidien. Delivering Energy Innovation: The New Cool-tip RF Ablaton System E Series. United Kingdom, 2011.
- Integra. Elektrotom HiTT 106 - Radiofrequency Thermoablation. Plainsboro, New Jersey, 2005.
- BostonScientific. LeVeen CoAccess Electrode System. Marlborough, Massachusetts, 2006:1-2.
- AngioDynamics I. RITA StarBurst Model 75, StarBurst SDE Electrosurgical Device: Instruction for Use. Manchester, GA, 2010.
- Livraghi T, Solbiati L, Meloni F, et al. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach”. Cancer 2003;97:3027-35. [PubMed]
- Nakamura S, Ishiyama M, Tsunoda-Shimizu H. Magnetic resonance mammography has limited ability to estimate pathological complete remission after primary chemotherapy or radiofrequency ablation therapy. Breast Cancer 2007;14:123-30. [PubMed]
- Nahirnyak VM, Moros EG, Novak P, et al. Doppler signals observed during high temperature thermal ablation are the result of boiling. Int J Hyperthermia 2010;26:586-93. [PubMed]
- Gillams AR, Lees WR. Radiofrequency ablation of colorectal liver metastases. Abdom Imaging 2005;30:419-26. [PubMed]
- Chadwick G, Groene O, Markar SR, et al. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett’s esophagus: a critical assessment of histologic outcomes and adverse events. Gastrointest Endosc 2014;79:718-731.e3.


