Intraoperative radiotherapy for breast cancer
Introduction
Interest in the role of accelerated partial breast irradiation (APBI) in the management of breast cancer has been growing. The rationale behind this approach is that the majority of local recurrences in the breast occur in the index quadrant whether radiotherapy is given or not. Therefore, “occult cancers” in other quadrants are probably not the cause of local recurrence and radiotherapy to the index quadrant alone maybe sufficient. The aim of APBI is to decrease the volume of breast irradiated whilst increasing the dose per fraction (hypofractionation).
A number of different techniques can be used for partial breast irradiation, including linear accelerator (LINAC)-based intensity modulated radiotherapy, interstitial brachytherapy, MammoSite and intra-operative radiotherapy (IORT). Although similar in principle, these techniques have large difference in dose rate and dose distribution, and are therefore not strictly comparable. IORT as a treatment for breast cancer is a relatively new method of delivering APBI that aims to replace whole breast external beam radiotherapy (EBRT) in selected women suitable for breast-conserving therapy.
IORT is possible because the development of mobile radiotherapy systems provides clinicians with the ability to readily provide treatment during the surgical intervention, as these systems can be taken into the operating rooms and used to irradiate the tumour bed immediately after the breast tumour (and surrounding margin of healthy tissue) has been removed. Mobile instruments include brachytherapy treatments using sealed sources (i.e., HDR), electron units (Mobetron, Novac-7) and photon units (Intrabeam™).
Targeted intraoperative radiotherapy (TARGIT) is a simple and straightforward IORT technique, the success of which depends on attention to detail and collaboration between the multidisciplinary team of surgeons, radiation oncologists, physicists, radiotherapy technicians and operating theatre and nursing staff. In brief, after excision of the tumour and a margin of healthy tissue, the tumour bed is mobilised to ensure that there is at least 5 mm distance between the surface of the applicator and the skin, in order to reduce the risk of radionecrosis. The equipment used is the Intrabeam™ system (Carl Zeiss Surgical, Oberkochen, Germany) which is a mobile, miniature X-ray generator where accelerated electrons strike a gold target at the tip of a 10 cm long drift tube resulting in the emission of low energy X-rays (50 kV) in an isotropic dose distribution. The irradiated tissue is kept at a fixed distance from the source by a spherical applicator to ensure a uniform dose distribution. A variety of sizes of applicators are available to fit the size of the surgical cavity; the dose rate depends on the diameter of the applicator with larger applicators requiring more time to deliver the dose of 20 Gy to the surface. Treatment times are typically between 20 to 45 minutes depending on the size of the applicator used. There is a steep attenuation of the radiation which allows the treatment to be safely carried out in unmodified operating theatres.
This article reviews twelve reasons for the use of IORT as treatment for breast cancer, with a particular emphasis on TARGIT.
Historical trends
EBRT is a safe and effective treatment after breast-conserving surgery, which reduces the in-breast recurrence rate by two-thirds (1) and the breast cancer death rate by about a sixth (2). However, with few exceptions, EBRT has been given to the entire breast. This is in contrast to surgery which has moved from radical (mastectomy) to minimal (lumpectomy) (3), but radiotherapy remains radical (whole breast), even though the results of many observational studies and clinical trials have demonstrated that around 90% of recurrent disease in the breast after breast conserving surgery is within the index quadrant, whether or not whole-breast EBRT has been given (4). Furthermore, after adjuvant endocrine therapy, the chance of a local recurrence outside the index quadrant is no more than the risk of a new contralateral tumour (5).
Timeliness of radiotherapy
Usually, EBRT is given several weeks after surgery, or several months if chemotherapy is also given. Several large population studies have shown that long intervals between breast conserving surgery and EBRT could be associated with inferior outcomes (6-8). By contrast, giving radiotherapy during primary surgery avoids any delay and may have beneficial biochemical effects that are important during the wound healing that occurs during recovery from surgery.
Evidence from translational research has found that treatment with TARGIT alters the molecular composition and biological activity of wound fluid in the tumour bed, which impairs the surgical trauma-stimulated proliferation and invasiveness of cultured breast cancer cells (9). Further work by the same group has found that treatment using the TARGIT technique modulates expression of microRNA that in turn modifies the expression of two growth factors known to be important in the regulation of cancer cell growth and motility, which may help to prevent local recurrences in early breast cancer (10).
Supported by level-one evidence
The international multicentre TARGIT A randomised controlled trial (ISRCTN34086741) was designed to determine non-inferiority between the TARGIT technique and conventional external beam radiotherapy (EBRT) in women with early breast cancer. IORT is given as a single dose of 20 Gy at the surface of the applicator (equivalent to 6 Gy at 1 cm) delivered directly to the tumour bed under direct observation. The primary outcome measure is local relapse within the treated breast; secondary endpoints are site of relapse within the breast, relapse-free and overall survival, and local toxicity/morbidity (11). The 5-year risk for local recurrence in the conserved breast when TARGIT was given concurrently with lumpectomy (n=2,298) had much the same results as EBRT: 2.1% (1.1-4.2) vs. 1.1% (0.5-2.5; P=0.31) (12).
A method of delivering IORT with electrons (instead of photons) has also reported results from a randomised controlled trial performed at the European Institute of Oncology (Milan, Italy). The results showed much poorer local control with this type of IORT when compared with EBRT: 35 patients in the IORT group and four patients in the external radiotherapy group had an ipsilateral breast tumour recurrence (P<0.0001). The 5-year event rate for IBRT was 4.4% [95% confidence interval (CI), 2.7-6.1] in the IOPT group and 0.4% (0-1.0) in the external radiotherapy group [hazard ratio 9.3 (95% CI, 3.3-26.3)] (13). This technique of delivering IORT, which is superficially similar to TARGIT, is in fact different in several important respects. Perhaps the most significant drawback of electron beam therapy is the inability to incorporate a risk-adjusted approach of giving EBRT after IORT to patients with additional risk factors. In the ELIOT trial, most of the local recurrences occurred in patients with disease characteristics suggesting subsequent use of whole breast irradiation (tumour >2.0 cm or ≥4 positive nodes or grade 3 or triple negative); patients without these characteristics had a much lower risk of local recurrence (1.7% 5-year event rate). There may therefore be a role for the Milan techniques of IORT using electrons during breast conserving surgery in a carefully selected population at low risk of local recurrence (14).
Regarding safety in the ELIOT study, information about side-effects of radiotherapy was available for 464 and 412 patients in the IORT group and EBRT group, respectively. Overall, skin side-effects showed a significant difference in favour of the IORT group (P=0.0002), with significantly reduced rates of erythema (P<0.0001), dryness (P=0.04), hyper-pigmentation (P=0.0004), and pruritus (P=0.002). The rate of fat necrosis was worse in the IORT group (P=0.04). In contrast, the TARGIT A trial reported that the frequency of any complications and major toxicity was similar in the IORT and EBRT groups [for major toxicity, TARGIT, 37 (3.3%) of 1,113 vs. EBRT, 44 (3.9%) of 1,119; P=0.44]. Radiotherapy toxicity (Radiation Therapy Oncology Group grade 3) was lower in the TARGIT group [six patients (0.5%)] than in the EBRT group [23 patients (2.1%); P=0.002] (15).
Two randomised trials of external partial breast irradiation (16,17) have not shown this technique to be an effective form of local control. A third trial (18), with a strict selection of patients, reported non-inferiority between partial and whole breast irradiation in terms of local control and survival after a median follow up of 10 years. A meta-analysis of partial breast irradiation (19) showed an increased risk for both local and regional recurrence with partial breast irradiation, with no survival difference, when compared with whole breast irradiation.
Risk-adaptive technique
It should be pointed out that IORT cannot always be given, even in women who meet the eligibility criteria. A study in Germany of 297 women found that in 55 women (19%) the planned IORT was not performed, mainly due to reasons which became apparent during surgery, namely: insufficient tumour-skin distance (n=20, 35.1%), an oversized wound cavity (n=14, 24.6%), and a combination of both (n=8, 14%) (20). In such cases, and in patients who have been given IORT, it is always possible to treat with EBRT at a later date, with omission of the tumour bed boost (if IORT has been given). This is why the technique is called “risk-adaptive”. Note that in such cases the patients do not “lose” anything, apart from a few more minutes under anaesthesia.
The replacement of the EBRT boost to the tumour bed by a TARGIT boost given during surgery is being tested in TARGIT-B (for boost, ISRCTN43138042), a multicentre randomised controlled trial that began accrual in 2013 (21). All patients will receive EBRT. The target population is patients with breast cancer who have a high risk of local recurrence. Specifically, patients should be either younger than 45 or, if older, need to have pathological features that confer a high risk of local recurrence of breast cancer, such as lymphovascular invasion, gross nodal involvement (not micrometastasis), more than one tumour in the breast but still suitable for breast-conserving surgery through a single specimen, ER negative, grade 3 histology or positive margins at first excision. This trial is based on results from a phase II non-randomised study in 299 unselected patients who had breast-conserving surgery and TARGIT as a boost to the tumour bed as a single dose of 20 Gy delivered intraoperatively. Postoperative external beam whole-breast radiotherapy excluded the usual boost. The treatment was well tolerated. After a median follow up of 60.5 months, eight patients had an ipsilateral recurrence, which gave a 5-year Kaplan-Meier estimate for ipsilateral recurrence of 1.73% (SE 0.77) (22). This therefore justifies the “risk adaptive” approach.
Reduced irradiation of normal tissues
In addition to the avoidance of irradiation of skin, the rapid attenuation of IORT X-rays means there is much less radiation exposure to normal tissues. An estimate was made of secondary cancer risks after IORT compared to APBI and EBRT by using computer-tomography scans of an anthropomorphic phantom with an Intrabeam™ IORT applicator in the outer quadrant of the breast; the scans were then transferred to a treatment planning system. For normal tissues, the maximal doses were calculated and lifetime risk for secondary cancers estimated. IORT delivered the lowest maximal doses to contralateral breast (<0.3 Gy), ipsilateral (1.8 Gy) and contralateral lung (<0.3 Gy), heart (1 Gy) and spine (<0.3 Gy). In comparison, maximal doses for APBI were 2-5 times higher. EBRT delivered a maximal dose of 10.4 Gy to the contralateral breast and 53 Gy to the ipsilateral lung. The estimated risk for secondary cancer was considerably lower after IORT and/or APBI as compared to EBRT (23).
Darby et al. (24) recently conducted a population-based case–control study of major coronary events (such as myocardial infarction, coronary revascularization, or death from ischemic heart disease) in women who received radiotherapy for breast cancer between 1958 and 2001. For each woman, the mean radiation dose to the heart was estimated. The overall average of the mean dose to the whole heart was 4.9 Gy; rates of major coronary events increased by 7.4% per gray (95% CI, 2.9 to 14.5; P<0.001), with no apparent threshold. It was concluded that exposure of the heart to ionizing radiation during radiotherapy increased the subsequent rate of ischemic heart disease. Brenner et al. (25) then performed a similar study on women exposed to contemporary (2005 and later) EBRT, and found a mean cardiac dose of 1.37 (95% CI, 1.12-1.61) Gy, less than one-third that found by Darby et al. However, the highest estimated radiotherapy-induced lifetime risks were still significant [3.52% (95% CI, 1.47-5.85%)]. It therefore seems that, even with modern radiotherapy planning techniques, EBRT can deliver an undesirable dose of radiation to the heart.
There is no question that TARGIT delivers less radiation to normal tissues. This has been demonstrated using a biological marker of radiation dose (gamma-H2AX in circulating lymphocytes) where it was shown that the DNA damage caused by X-radiation was significantly less with TARGIT compared with EBRT (26).
Better cosmetic outcome
The use of IORT has been shown to result in better cosmetic outcome when compared with EBRT (27) using an objective evaluation of aesthetic outcome. Frontal digital photographs were taken at baseline (before TARGIT or EBRT) and annually thereafter for up to 5 years. The photographs were analysed by a validated, specialised software application that produces a composite score (Excellent, Good, Fair, Poor) based on symmetry, colour and scar. There was a statistically significant increases in the odds of having an outcome of Excellent or Good for patients in the TARGIT group relative to the EBRT group at year 1 [odds rate (OR) 2.07, 95% CI, 1.12-3.85, P=0.021] and year 2 (OR 2.11, 95% CI, 1.0-4.45, P=0.05). This study demonstrated that women treated with TARGIT had a superior cosmetic result compared with patients who received conventional EBRT.
The cosmetic effects of breast conservation therapy have been studied for decades, but the usual methods for evaluating cosmetic outcome are assessments made by the clinical care team, including the surgeon who performed the operation. Although this is an important source of feedback, such information is obviously biased and cannot be used reliably to compare techniques carried out by different clinicians, perhaps at different centres, using different techniques. Some researchers utilise a blinded assessment of outcome, usually by an independent panel examining photographs, but this is time consuming and too cumbersome to be used in routine practice.
There are few published studies on the measurement of cosmesis after IORT. In the MSKCC Series (28), where quadrant IORT of 18-20 Gy was given, the cosmetic outcome was acceptable. In the Montpellier phase II trial (29) IORT was given as electrons (21 Gy); at a median follow-up of 30 months of 94 patients, all showed excellent or good cosmesis. In a study of IORT using Axxent, a balloon-based electronic brachytherapy (20 Gy), at median follow-up of 12 months there was excellent cosmesis in 10 of the 11 patients (30). However, an interim report from a large randomised controlled trial found that APBI increased rates of adverse cosmesis (and late radiation toxicity) compared with EBRT (31).
Economically viable
The institutional perspective
An economic evaluation of new treatments is a key part of a health technology assessment, as resources are finite. Appleby has shown that, in the past 30 years, total national expenditures on healthcare as a percentage of gross domestic product (GDP) have increased. In the UK this figure rose from 3.9% in 1960 to 9.4% in 2010, in Germany it rose from 6.0% in 1970 to 11.6% in 2010 and in the US from 5.1% in 1960 to 17.6% in 2010 (32).
The population is ageing; the demand on LINAC units for treatment of breast cancer is approximately a third of the workload in the UK, and rising, putting an increasing burden on radiotherapy infrastructure. For IORT, one consideration is the capital cost to purchase the required equipment, which is at least one-tenth of that required for a LINAC capable of delivering EBRT. This is without taking into account the requirements for shielding the LINAC (which usually requires significant amounts of shielding, often in the form of a “concrete bunker”); with TARGIT, minimal shielding is required in the operating theatre (33). It might be argued that as the institution has already invested in the LINAC, it might as well use it. However, workload on radiotherapy departments is increasing, so alternative methods of delivering radiotherapy should be considered, particularly those that are safe, efficacious, and cost-effective.
A Markov decision-analytic model developed in the USA has shown that significant cost savings are possible when using TARGIT even if only a small proportion of women with early breast cancer are offered IORT as opposed to EBRT (34). IORT single-dose intraoperative radiation therapy was the more cost-effective strategy, providing greater quality-adjusted life years at a decreased cost. The authors concluded that IORT offers a unique example of new technology that is less costly than the current standard of care option but offers similar efficacy; the capital investment for the equipment could be recouped after 3-4 years.
However, Shah et al. (35), using cost-minimization analyses, compared intra-operative radiation therapy (IORT) with whole-breast irradiation (WBI) and accelerated partial-breast irradiation (APBI) and concluded that APBI and WBI are cost-effective compared with IORT. This was partially due to the level of reimbursement paid in the USA for these treatments.
Another economic consideration is the additional time required in the operating theatre for delivery of the radiation. In some circumstances, this can be offset by using this time for the intraoperative testing of the histological status of the sentinel lymph nodes, as most patients who undergo IORT also require sentinel node biopsy. An example is the one stage nucleic acid (OSNA) test. During administration of the IORT the sentinel lymph node is processed; by the end of treatment, the result of the OSNA test has been received and further surgery can be performed if required. It is therefore possible for a woman to have complete removal of the tumour, clearance of the axilla (if necessary), and radiotherapy all in a single session.
Another economic argument is that the IORT equipment can be used for applications other than breast cancer, such as treatment during kyphoplasty for vertebral metastases (36), or to deliver intravaginal radiotherapy (37).
Societal perspective
This perspective is wide and explores costs and benefits borne by all. The IORT equipment does not need to be based in a radiotherapy centre; it can be used in any operating theatre. Also, IORT is given as a single fraction, and does not require daily visits over three to five weeks. These factors mean that patients may have reduced travel time for treatment, and reduced time off work (or as primary carers for family). A large study in the USA found that women traveling over 75 km for treatment are about 1.4 times more likely to receive a mastectomy than those traveling under 15 km (38).
These factors need to be further explored to tease out not only costs saved by patients due to reduced travel time and reduced time off work, but also to interrogate the effects of shifting radiotherapy burden away from radiotherapy centres and into surgical theatres, effects on income tax, works and pensions, savings to families and employers, and effects on private insurance premiums and claims. A full assessment would yield a “treasure trove” of information.
Excellent patient preference and satisfaction
A study in the USA (39) used a trade-off technique to vary the risk of local recurrence for IORT and quantify any additional hypothetical 10-year local recurrence risk that patients would accept to receive either IORT or EBRT. Data from 81 patients showed that the median additional accepted risk to have IORT was 2.3% (from 9% to 39%), mean 3.2%. These results demonstrate that the majority of women with breast cancer will accept a small increment of local risk for a simpler delivery of radiation.
A study in Australia compared preferences of women after they had received either IORT or EBRT. There was discordance in the willingness of patients to accept additional risk; the patients who had received EBRT were risk-averse, whilst patients who had received IORT valued the convenience of this treatment: 60% of them would accept an additional risk of recurrence as high as 4% to 6% (40).
Better quality of life
It should be noted that although patient-reported outcome measures are increasingly used and quality of life is a key measure of clinical effectiveness, the measures used fall short of those required for evidence-based medicine. A review of 227 outcome studies for aesthetic and reconstructive breast surgery found only one study that was validated, specific and reproducible (41). The use of objective measurement of the patient’s perception and expectations is needed to assist in the development of accurate predictive tools to better enable clinicians and patients to choose the optimal treatment.
It has been demonstrated in Germany that women who received IORT had superior radiation-related quality of life parameters compared with those who have EBRT (42). A single-centre subgroup of 87 women from the two arms of the randomized controlled TARGIT-A trial found that patients receiving IORT alone reported less general pain, fewer breast and arm symptoms, and better role functioning than patients receiving EBRT (P<0.01).
Quality of life has also been reported to be high during/after MammoSite breast brachytherapy (43).
IORT can be given to a previously irradiated breast
It is possible to apply IORT to a breast that has already been exposed to whole-breast EBRT, provided that the woman is suitable for a second breast-conserving procedure (44). This means that such women can be given an alternative to salvage mastectomy.
Furthermore, there is limited but encouraging experience of giving IORT twice within the same breast, for the primary cancer and a subsequent new primary cancer in another quadrant that developed some years later (H Flyger, Denmark, personal communication).
IORT can be given to women who would not be given EBRT
There are women who present with special circumstances who would never be considered for EBRT and for whom mastectomy is the only option; such as those who are frail and elderly, or who have Parkinson’s Disease, or who have a cardiac pacemaker, or have collagen vascular disease. A study performed on a group of such patients has shown that IORT is an option that should be considered in such patients (45). Thirty-one patients were treated with TARGIT due to clinical reasons for not receiving EBRT such as systemic lupus erythematosus, motor neuron disease, Parkinson’s disease, ankylosing spondylitis, morbid obesity, and cardiovascular or severe respiratory disease. A further 28 patients were included for compelling personal reasons, usually on compassionate grounds. After a median follow-up of 38 months, only two local recurrences were observed, an annual local recurrence rate of 0.75% (95% CI, 0.09-2.70%). This evidence suggests that TARGIT is an acceptable option in highly selected cases in whose EBRT is not feasible or possible.
The influence of age on short-term complications in women undergoing IORT for early breast cancer was investigated in a retrospective study of 188 women who underwent IORT during breast-conserving surgery and found that acute toxicity after IORT in women aged 70 years and older was not higher compared to younger patients (46).
TARGIT-E (for elderly) is a single-arm trial on use of TARGIT in elderly patients being run in Mannheim by Professor Frederik Wenz. The protocol is based on the international TARGIT-A study. The purpose is to investigate the efficacy of a single IORT treatment within elderly low-risk patients (≥70 years, cT1, cN0, cM0, invasive ductal carcinoma) which is followed by EBRT only when adverse risk factors are present (47).
TARGIT Academy
Although the TARGIT IORT technique is relatively straightforward, the success of this technology requires adequate training and attention to detail. The lessons learned from the introduction of new surgical techniques in surgery in the past have been appreciated and incorporated into a unique training scheme.
The TARGIT Academy was established in 2010 and is co-directed by Professor Mo Keshtgar from the Royal Free and University College London, UK and Professor Frederik Wenz from the University Medical Centre Mannheim & University of Heidelberg. The remit of the TARGIT Academy is to offer high quality training together with the opportunity to gain access to a broad academic network. The faculty are from a variety of disciplines, many of whom have been involved with this technology from the outset. Some of the trainers have extensive experience in performing the procedure and can provide “first hand” advice for difficult cases that are seen occasionally. Regular training courses are run in London and Mannheim and are sponsored by the manufacturer of the Intrabeam™ (Carl Zeiss Surgical, Oberkochen, Germany).
The foundation of the TARGIT Academy is a networking platform, which enables interaction and cooperation between surgeons, radiation oncologists, medical physicists and other members of the multidisciplinary team. As the success of this technique depends on multidisciplinary team working harmoniously with each other, the training is available only to teams and not individuals. At the outset it was felt important that the entire team should listen to some of the detailed specialist instructions so that they can appreciate the roles of their colleagues more fully. For example, many of the surgeons will be unfamiliar in the use and handling of the IORT device, selection of an appropriate applicator and adequate placement in the tumour bed; it is important that the entire team are aware of these “surgical” details, as they can have a bearing on other aspects of patient management.
The UK Newstart sentinel node biopsy training programme (48) has been used as a model for the TARGIT Academy. The training involves three phases: theory and hands on experience in a skills laboratory; proctored training; and an audit phase.
Theory and hands-on training
The TARGIT Academy offers participants a unique first hand education and intensive hands-on training, which accelerates the learning curve to the optimum level within shortest time. An extensive training course is run over two days, and is intended to provide peer-led training about proper selection of patients, current clinical trial results, the safe use of Intrabeam™ and the precise use of the TARGIT technique. There is also emphasis on the radiobiology and radiation safety aspects of this procedure to ensure correct guidelines are followed. As the indications for the use of TARGIT is expanding to other tumour sites, these new developments are also covered. At the end there is a group discussion, incorporating troubleshooting and presentations of interesting cases in a mock multidisciplinary meeting setting.
Educational material
The educational material comprises of full slide set of the training programme, a video recording of the procedure performed in the operating theatre, and a comprehensive reference list with copies of key publications. All participants receive a copy of a comprehensive textbook edited by the course organisers (49), which has recently been revised and updated (50).
The TARGIT training simulator
In order for the surgeons and the interdisciplinary team to be equipped with the appropriate practical skills required to perform the procedure, a bespoke training simulator, conceived by one of the authors (MK), has been designed and built, and is commercially available (51). This simulator accelerates the learning curve to the optimum level within the shortest time, as it is realistic anatomically, and is constructed from a hot-melt thermoplastic polymer with similar physical characteristics and radiation attenuation qualities to the human breast, which therefore allows an accurate demonstration of the technical aspects of TARGIT. Simulated tumours of different sizes and locations are implanted within the model to simulate a real life experience as far as possible. The polymer can be cut with a scalpel, and stitches applied. The design of the simulator is such that participants get an opportunity to practice the implementation of the entire treatment workflow. This is accompanied by an in-depth demonstration of the practical aspects by reviewing the recorded operative procedure on an actual patient (see Figures 1 and 2).

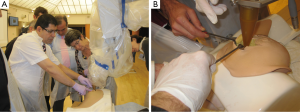
Proctored training
The Academy also facilitates proctored training for TARGIT naïve centres that have recently acquired the equipment. This is done by an experienced trainer and provides an opportunity for the extended multidisciplinary team including the operating theatre staff to familiarize themselves with the technical aspects of the procedure. In order to ensure that adequate numbers of trainers are available, a “training the trainer” programme is being designed and will commence soon.
Audit phase
Centres that intend to participate in one or more of the international TARGIT trials need to complete an audit phase by successfully performing five TARGIT procedures, followed by review and approval by the trial steering group. This ensures uniformity of practice within the participating centres in the trial.
TARGIT Academy website
A TARGIT Academy website (52) is an interactive, up-to-date portal with both “open” and “closed sections which can only be accessed by the members of the academy. The intention is to develop a group discussion forum so that staff from centres all over the world can look up new information, or refresh their memories of information that they may have forgotten. All the educational materials and latest developments on TARGIT will be posted on the website for access by Intrabeam™ users.
There has been a significant interest in the TARGIT technique since the first publication of the results of the randomized controlled trial confirming its safety and efficacy. It is essential that as new centres take up this innovative technology worldwide, there is an adequate quality assurance and training program in place to ensure that this powerful technology is implemented appropriately. The establishment of the TARGIT Academy is a step towards achieving this objective.
Conclusions
Finally, it should be mentioned that TARGIT is the only IORT technique that is capable of delivering X-rays within the operating theatre, and is supported by level-one evidence. In addition to the trials already mentioned, the following studies will continue to gather high-quality evidence for the effectiveness of the technique.
TARGIT-US (for United States) is a phase IV registry trial being run in the USA through the University of California, San Francisco to study the efficacy and toxicity of breast radiotherapy given intraoperatively as a single dose after breast-conserving surgery, with or without whole breast radiation as indicated by pathological risk factors, in women with early stage breast cancer (53).
TARGIT-R (for registry, ISRCTN91179875) will be an open registry study with very wide inclusion criteria, enabling clinicians to treat patients with the TARGIT technique provided they have the support of their institution and colleagues. Data collection will be as per the existing TARGIT-A trial and is ideal for centres involved in this trial who wish to continue to treat patients now that randomisations have ceased (54).
Acknowledgements
All authors made substantial contributions to the conception, design and interpretation of data for this article; and drafted the article and revised it critically for important intellectual content; and gave final approval of the version to be published; and agree to be accountable for all aspects of the article and will ensure that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Funding: The authors wish to acknowledge the TARGIT International Steering Committee, NIHR HTA (for funding the TARGIT A and B trials), and David Bishop (for medical photography).
Disclosure: The authors declare no conflict of interest.
References
- Vinh-Hung V, Verschraegen C. Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst 2004;96:115-21. [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. [PubMed]
- Keshtgar M, Davidson T, Pigott K, et al. Current status and advances in management of early breast cancer. Int J Surg 2010;8:199-202. [PubMed]
- Williams NR, Pigott KH, Keshtgar MR. Intraoperative radiotherapy in the treatment of breast cancer: a review of the evidence. Int J Breast Cancer 2011;2011:375170.
- Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996;14:2738-46. [PubMed]
- Olivotto IA, Lesperance ML, Truong PT, et al. Intervals longer than 20 weeks from breast-conserving surgery to radiation therapy are associated with inferior outcome for women with early-stage breast cancer who are not receiving chemotherapy. J Clin Oncol 2009;27:16-23. [PubMed]
- Mikeljevic JS, Haward R, Johnston C, et al. Trends in postoperative radiotherapy delay and the effect on survival in breast cancer patients treated with conservation surgery. Br J Cancer 2004;90:1343-8. [PubMed]
- Hershman DL, Wang X, McBride R, et al. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys 2006;65:1353-60. [PubMed]
- Belletti B, Vaidya JS, D’Andrea S, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res 2008;14:1325-32. [PubMed]
- Belletti B, Massarut S, D’Andrea S, et al. P259 TARGIT modulates miRNAs expression to control growth factors production in breast tissue. Breast 2011;20:S62.
- National Institute for Health Research Evaluation Trials and Studies Coordinating Centre [Internet]. Southampton [cited 2014 Mar 18]. HTA - 07/60/49: An international randomised controlled trial to compare targeted intraoperative radiotherapy (TARGIT) with conventional post-operative radiotherapy for women with early breast cancer; [about 2 screens]. Available online: http://www.nets.nihr.ac.uk/projects/hta/076049
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [PubMed]
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. [PubMed]
- Azria D, Lemanski C. Intraoperative radiotherapy for breast cancer. Lancet 2014;383:578-81. [PubMed]
- Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91-102. [PubMed]
- Dodwell DJ, Dyker K, Brown J, et al. A randomised study of whole-breast vs tumour-bed irradiation after local excision and axillary dissection for early breast cancer. Clin Oncol (R Coll Radiol) 2005;17:618-22. [PubMed]
- Ribeiro GG, Magee B, Swindell R, et al. The Christie Hospital breast conservation trial: an update at 8 years from inception. Clin Oncol (R Coll Radiol) 1993;5:278-83. [PubMed]
- Polgár C, Fodor J, Major T, et al. Breast-conserving therapy with partial or whole breast irradiation: ten-year results of the Budapest randomized trial. Radiother Oncol 2013;108:197-202. [PubMed]
- Valachis A, Mauri D, Polyzos NP, et al. Partial breast irradiation or whole breast radiotherapy for early breast cancer: a meta-analysis of randomized controlled trials. Breast J 2010;16:245-51. [PubMed]
- Tuschy B, Berlit S, Nasterlack C, et al. Intraoperative radiotherapy of early breast cancer using low-kilovoltage x-rays-reasons for omission of planned intraoperative irradiation. Breast J 2013;19:325-8. [PubMed]
- National Institute for Health Research Evaluation Trials and Studies Coordinating Centre [Internet]. Southampton [cited 2014 Mar 18]. HTA - 10/104/07: TARGIT-B: An international randomised controlled trial to compare targeted intraoperative radiotherapy boost with conventional external beam radiotherapy boost after lumpectomy for breast cancer in women with a high risk of local recurrence; [about 2 screens]. Available online: http://www.nets.nihr.ac.uk/projects/hta/1010407
- Vaidya JS, Baum M, Tobias JS, et al. Long-term results of targeted intraoperative radiotherapy (Targit) boost during breast-conserving surgery. Int J Radiat Oncol Biol Phys 2011;81:1091-7. [PubMed]
- Aziz MH, Schneider F, Clausen S, et al. Can the risk of secondary cancer induction after breast conserving therapy be reduced using intraoperative radiotherapy (IORT) with low-energy x-rays? Radiat Oncol 2011;6:174. [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [PubMed]
- Brenner DJ, Shuryak I, Jozsef G, et al. Risk and Risk Reduction of Major Coronary Events Associated With Contemporary Breast Radiotherapy. JAMA Intern Med 2014;174:158-60. [PubMed]
- Woolf DK, Williams NR, Bakhshi R, et al. An observational study using gamma-H2AX foci to investigate cardiac doses of radiation in women following adjuvant radiotherapy for breast cancer: External beam radiotherapy versus targeted intraoperative radiotherapy. San Antonio Breast Cancer Symposium, 2013.
- Keshtgar MR, Williams NR, Bulsara M, et al. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treat 2013;140:519-25. [PubMed]
- Sacchini V, Beal K, Goldberg J, et al. Study of quadrant high-dose intraoperative radiation therapy for early-stage breast cancer. Br J Surg 2008;95:1105-10. [PubMed]
- Lemanski C, Azria D, Gourgon-Bourgade S, et al. Intraoperative radiotherapy in early-stage breast cancer: results of the montpellier phase II trial. Int J Radiat Oncol Biol Phys 2010;76:698-703. [PubMed]
- Ivanov O, Dickler A, Lum BY, et al. Twelve-month follow-up results of a trial utilizing Axxent electronic brachytherapy to deliver intraoperative radiation therapy for early-stage breast cancer. Ann Surg Oncol 2011;18:453-8. [PubMed]
- Olivotto IA, Whelan TJ, Parpia S, et al. Interim cosmetic and toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol 2013;31:4038-45. [PubMed]
- Appleby J. Rises in healthcare spending: where will it end? BMJ 2012;345:e7127. [PubMed]
- Eaton DJ, Gonzalez R, Duck S, et al. Radiation protection for an intraoperative X-ray device. Br J Radiol 2011;84:1034-9. [PubMed]
- Alvarado MD, Mohan AJ, Esserman LJ, et al. Cost-effectiveness analysis of intraoperative radiation therapy for early-stage breast cancer. Ann Surg Oncol 2013;20:2873-80. [PubMed]
- Shah C, Badiyan S, Khwaja S, et al. Evaluating Radiotherapy Options in Breast Cancer: Does Intraoperative Radiotherapy Represent the Most Cost-Efficacious Option? Clin Breast Cancer 2014;14:141-6. [PubMed]
- Reis T, Schneider F, Welzel G, et al. Intraoperative radiotherapy during kyphoplasty for vertebral metastases (Kypho-IORT): first clinical results. Tumori 2012;98:434-40. [PubMed]
- Schneider F, Fuchs H, Lorenz F, et al. A novel device for intravaginal electronic brachytherapy. Int J Radiat Oncol Biol Phys 2009;74:1298-305. [PubMed]
- Boscoe FP, Johnson CJ, Henry KA, et al. Geographic proximity to treatment for early stage breast cancer and likelihood of mastectomy. Breast 2011;20:324-8. [PubMed]
- Alvarado MD, Conolly J, Park C, et al. Patient preferences regarding intraoperative versus external beam radiotherapy following breast-conserving surgery. Breast Cancer Res Treat 2014;143:135-40. [PubMed]
- Corica T, Nowak A, Saunders C, et al. Patient Preferences for Adjuvant Radiotherapy in Early Breast Cancer - an Australian Sub-study of the International TARGIT Trial. Eur J Cancer 2012;48:S187.
- Pusic AL, Chen CM, Cano S, et al. Measuring quality of life in cosmetic and reconstructive breast surgery: a systematic review of patient-reported outcomes instruments. Plast Reconstr Surg 2007;120:823-37. [PubMed]
- Welzel G, Boch A, Sperk E, et al. Radiation-related quality of life parameters after targeted intraoperative radiotherapy versus whole breast radiotherapy in patients with breast cancer: results from the randomized phase III trial TARGIT-A. Radiat Oncol 2013;8:9. [PubMed]
- Dragun AE, Harper JL, Taylor CE, et al. Patient satisfaction and quality of life after MammoSite breast brachytherapy. Am J Surg 2008;196:545-8. [PubMed]
- Kraus-Tiefenbacher U, Blank E, Wenz F. Intraoperative radiotherapy during a second breast-conserving procedure for relapsed breast cancer after previous external beam radiotherapy. Int J Radiat Oncol Biol Phys 2011;80:1279-80. [PubMed]
- Keshtgar MR, Vaidya JS, Tobias JS, et al. Targeted intraoperative radiotherapy for breast cancer in patients in whom external beam radiation is not possible. Int J Radiat Oncol Biol Phys 2011;80:31-8. [PubMed]
- Tuschy B, Berlit S, Romero S, et al. Influence of age on short-term complications after intraoperative radiotherapy in women after breast-conserving surgery. Anticancer Res 2013;33:3995-9. [PubMed]
- National Institutes of Health [Internet]. Bethesda [updated 2011 Feb 18; cited 2014 Mar 18]. Prospective Phase II Study of Intraoperative Radiotherapy (IORT) in Elderly Patients With Small Breast Cancer (TARGIT-E); [about 2 screens]. Available online: http://www.clinicaltrials.gov/ct2/show/NCT01299987?term=targit+e&rank=1
- Mansel RE, MacNeill F, Horgan K, et al. Results of a national training programme in sentinel lymph node biopsy for breast cancer. Br J Surg 2013;100:654-61. [PubMed]
- Wenz F, Kraus-Tiefenbacher U. eds. Intraoperative Radiotherapy for Breast Cancer. 1st edition. UNI-MED Science, 2011.
- Keshtgar M, Pigott K, Wenz F. eds. Targeted Intraoperative Radiotherapy in Oncology. Springer, 2014.
- Pharmabotics Ltd. [Internet]. c2010 [cited 2014 Mar 19]. Available online: http://www.pharmabotics.com/
- TARGIT Academy [Internet]. c2012-1014 [updated 2014 Mar 17; cited 2014 Mar 19]. Available online: http://www.targitacademy.com/
- National Institutes of Health [Internet]. Bethesda [updated 2014 Jan 30; cited 2014 Mar 18]. Targeted Intraoperative Radiotherapy United States (TARGIT-US) Registry Trial; [about 2 screens]. Available online: http://www.clinicaltrials.gov/ct2/show/NCT01570998?term=targit&rank=4
- International Standard Randomised Controlled Trial Number Register [Internet]. Current Controlled Trials Ltd. [updated 2013 Nov 15; cited 2014 Mar 19]. TARGIT R: TARGeted Intraoperative radioTherapy (TARGIT) Registry database; [about 2 screens]. Available online: http://www.controlled-trials.com/ISRCTN91179875/targit


