Curative versus palliative surgical resection of liver metastases in patients with neuroendocrine tumors: a meta-analysis of observational studies
Introduction
Neuroendocrine tumors (NETs) are a group of neoplasms with different clinical presentation and growth rates (1). Historically, NETs have been considered as rare tumors, representing approximately 0.5% of all malignant conditions. More recent series incorporating large prospective tumor registries (SEER) report on the linearly increasing overall incidence and changing spectrum of NET manifestation over the past four decades. Even being considered benign it is common to find these tumors in advanced stage often with liver metastases (2-7). Different medical and surgical treatments have been proposed for patients with liver metastases from NETs (3,8-10). However, the exact role and real effectiveness of surgery for patients with liver NETs metastasis is still poorly defined considering, the frequent slow growth and long-term natural history of these tumors. This review was made to evaluate the survival impact of liver resection in patients with hepatic metastases NET’s tumors.
Materials and methods
Search strategy for review
Three authors independently carried out a literature search by gathering information from Medline, Embase, Ovid, Google Scholar, and Cochrane database for studies published form January 1990 to October 2013. Search terms included “neuroendocrine tumor” or “carcinoid tumor” or “gastrointestinal NETs” or “liver metastases” or “hepatic metastases” or “neuroendocrine metastases” and “hepatectomy” or “liver resection” or “liver transplantation”. All the titles and the abstracts resulted from these queries were examined. We first selected all the articles that referred to the surgical treatment of liver metastases from NETs. Second, we analyzed the full articles. Third, we used bibliographies and citations from full articles and previous review publications to identify other additional pertinent articles.
Inclusion and exclusion criteria
All observational and experimental studies that evaluated survival in patients affected by NET liver metastases and treated by hepatic complete surgical resection or palliative surgical resection were considered. All included studies were observational (level III or IV of evidence, CEBM) (11) and no randomized trials comparing complete or palliative resections of liver metastases have been found. We considered, in this meta-analysis, Kaplan-Meier curves or Cox proportional hazards regression models to calculate the survival difference among patients treated with palliative or complete resection of liver metastases. Moreover, we included only articles with the full text available for data retrieval that were performed on human subjects and written in English. We retrieved from full text articles time frame for NET diagnosis, geographic locations, and treatment in order to avoid any possible population overlap. The study of better quality or with more detailed data was included in case of two or more studies presenting possible data overlap. When discrepancies among the three reviewers were found, a joint reevaluation of the original article was performed to address them. Studies considering <20 patients, non-English written articles, or studies about nonhuman subjects were specific exclusion criteria. In addition, letters to the editor without original data, editorials, case reports, and reviews were excluded. Moreover, conference abstracts due to the lack of details regarding survival data and study design were excluded.
Data extraction
Three independent reviewers extracted data from the selected articles by using a predefined data extraction form. As previously described, any discrepancies in data extraction or unsuitability for inclusion were discussed (12) and the following information was extracted: authors, year of publication, geographical area, population characteristics (age, sex, etc.), study design, number of patients, type of procedure applied, hazards ratios with 95% confidence interval (95% CI), or hazards ratios (HRs) extracted from Kaplan-Meier curves. The HR was calculated using methods previously described from data obtained from published reports (13).
Quality assessment for included studies
The quality of each included study was assessed by using the Newcastle-Ottawa Scale. We defined studies of high quality those that scored nine or eight points on the Newcastle-Ottawa Scale and studies of medium quality those that scored seven or six points. Discrepancies in quality assessment were solved as previously described (12).
Data analysis
Data was analyzed by R (version 3.0.1), considering significant the P<0.05. We calculated a summary statistic considering the hazards ratio for survival analysis. Rank correlation test of funnel plot asymmetry was used to test the presence of any publication bias (14,15). The I2 index and the Cochran Q to assess the heterogeneity among studies were used. As previously described an I2 index value >50% and, a Q statistic P value <0.10 were considered statistically significant signs for heterogeneity (16). We applied, where appropriate the fixed- and the random-effect model to calculate the pooled estimate. We reported the primary outcome in this meta-analysis as HR (with 95% CI) of overall survival in patients treated with complete hepatic resection of metastases. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines for accurate performing meta-analysis of observational studies (17) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines checklist (18) were considered to prepare this meta-analysis.
Results
Search results
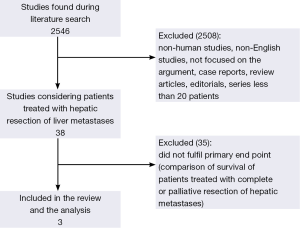
During the first stage of literature search we found 2,546 studies (Figure 1). In Figure 1 is shown the literature search design: after reviewing the titles and abstracts we found 2,259 articles to be not eligible as they were case reports, review articles, editorials, non-human studies or non-English articles, not focusing on the review topic, and others not meeting the inclusion criteria. A total of 38 articles as potentially eligible for this review were identified. A total of 35 of these articles either did not described the outcome differences between complete hepatic resections and palliative surgery (5,9,19-30) or did not reported any HR (31-41) or Kaplan-Meier curves to compare complete surgical resection of hepatic metastases with palliative resection of hepatic metastases (42-52). Therefore they were excluded. We finally selected three eligible articles that compared survival between patients treated by curative surgical resection of hepatic metastases and patients treated by palliative debulking surgery using Kaplan-Meier curves (Figure 1) (53-55). These included research articles were found to be all observational retrospective studies.
Characteristics of the studies
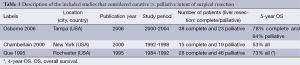
In this meta-analysis we included three retrospective observational studies that evaluated survival in patients affected by NET comparing curative surgical resection of hepatic metastases with palliative surgical resection (Table 1) (53-55). All the three included studies compared curative/complete surgical resection with incomplete/palliative surgical resection.
Full table
In the paper of Osborne et al., surgical exploration was undertaken when resection of at least 90% of the hepatic disease was feasible. In this study, the resections were defined as curative if the entire hepatic disease was removed or palliative if there was, after resection, evidence of gross residual disease. Tumors that were ablated with radiofrequency were considered curative when all gross disease was addressed. Osborne et al. compared also incomplete/palliative surgical resection of liver metastases with conservative treatments (embolisation) and they found that the survival difference when comparing palliative cytoreduction with embolization was significant (53).
In the analysis of Que et al., resections were considered curative only if all gross primary, regional and hepatic extent, was resected and palliative when gross residual disease was present either intra-abdominally at operation or extra-abdominal by imaging.
Chamberlain et al. stated that resections were considered curative only if all gross disease was resected. In the other cases, palliative hepatic surgery for symptoms palliation was undertaken.
In Table 1 the characteristics of the included studies are shown. The HR was extracted from Kaplan-Meier curves because none of the three included studies presented Cox proportional hazards multivariate regression models. All the excluded studies were observational (5,9,19-30) and retrospective (31-41) and HR extraction was not possible (42-52). The majority of these excluded studies were described and summarized by recent review articles (12,56).
Quality assessment of the included studies
The quality of the evidence about the influence of complete surgical resection in comparison to incomplete resection on survival of patients with NET liver metastases is low (levels III-IV, CEBM) (11).
All studies in our meta-analysis showed an increased survival in the groups treated with curative surgical resection of liver metastases but none of the included studies was randomized. The three independent reviewers agreed that all studies were graded seven or six points according to Newcastle-Ottawa scale for quality (medium quality).
Main analysis
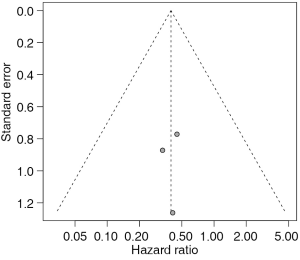
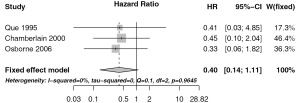
The meta-analysis was performed on the three selected studies, as shown in Figure 2. The included studies analyzed a total amount of 169 patients affected by NET liver metastases: 81 treated by radical surgery and 88 by palliative surgery. The I2 index value was 0% and the Q statistic P value was 0.965 therefore we found no heterogeneity among the included studies and the fixed-effect model to calculate the pooled estimate was used. We found an increased survival, but not statistically significant, in the group of patients treated with complete surgical resection of hepatic metastases HR 0.40 (95% CI: 0.14-1.11) compared with patients who underwent only palliative resection (Figure 2).
Risk of bias assessment
All the included observational studies were classified as medium quality ones. The main limit to consider observational retrospective studies was that of a possible selection bias of different authors in considering patients suitable for curative surgery or palliative surgery of liver metastases. The article of Que et al. did not highlight the differences between the two groups (curative vs. palliative surgical resection), and it is also unclear whether in cases of palliative surgery the non-radical resection refers to the primitive tumor or the liver metastasis (55). In the article of Chamberlain et al., there were no differences between radical and palliative surgical resection of liver metastases with regard to gender, age, synchronous or metachronous presentation, tumor histology or location, indications for treatment, hormone secretion, or percentage of liver involvement. However, the two studied groups did significantly differ in terms of lobar liver involvement and the majority of bilobar disease involvement underwent palliative surgical resection of liver metastases (54). The study of Osborne et al. did not showed the possible differences in pre-treatment status between radical and palliative surgical resection of liver metastases (53).
Publication bias
The presence of a possible publication bias was examined (Figure 3). These results should be considered with caution, because our meta-analysis calculation included only three studies (Figure 2). Anyway, none of the studies seems to be out of the symmetry in the plot (Figure 3). As a consequence, the rank correlation test of funnel plot asymmetry had a P value of 0.908. However, the current guidelines do not recommend testing for the funnel plot asymmetry or for the correlation test of funnel plot asymmetry in analysis of a limited number of studies (<10) (57,58).
Discussion
Recent data demonstrate that the incidence of NETs has increased exponentially (overall 500%) over the last three decades (59). In an analysis on 13,715 patients, Modlin stated that in 12.9% of all carcinoid patients, distant metastases were already evident at the time of diagnosis and the overall 5-year survival rate for all carcinoid tumors was 67.2% (60). These findings bring into question the widely promulgated benignity of these neoplasms and now many clinicians think that all NETs should be considered to have potential malignancy (61-65). The occurrence of hepatic metastases is one of the most important prognostic factors for NET’s survival (3,42,54,66), but still now, considerable controversy exists concerning how to approach patients with neuroendocrine metastases. The management of these patients varies from control of symptoms to more aggressive surgical or radiological therapies. For patients with non-resectable liver disease, treatments like bio-therapy with somatostatin analogues, radio-peptide receptor therapy, transarterial chemoembolization, selective intra-arterial radiotherapy or new molecular target-directed therapy can be performed (67-69). For localized hepatic metastases, surgical therapy appears the most efficient approach (8,9,27,42,66,70-72). A potential curative resection of liver metastases can be undertaken in 13.7-24.5% of the patients with advanced NETs (28,45,73). Symptomatic patients can be relieved with cytoreductive procedures, but the role of surgery, particularly if aggressive, is more debated for patients with asymptomatic disease. To complicate the problem of the best therapy for patients with metastatic NETs, many studies reporting the outcome following surgical management of liver metastases focused solely on resection rather than combined-modality approaches.
The survival impact of hepatic resection is difficult to assess also for other reasons. First, the patient selection criteria differ in many centers and, in several studies, the completeness of resection was not clearly determined. Second, most studies give analysis of pooled data of uncleared primary site of NETs from forgut, midgut and hindgut origin. Third, the results of surgery or other therapies have often not been determined separately in the absence of prospective randomized controlled trials (RCTs) and therefore recommendations have to rely only on recently published retrospective series.
The published studies suggest that surgical resection of hepatic neuroendocrine neoplasms can be associated with favorable survival, but clinical and oncologic variables that can distinguish patient cohorts taking advantage from such aggressive therapy have not been identified. Also being not available RCTs evaluating patients with liver metastases from NETs, the question of effectiveness of other treatment modalities such as RFA, chemoembolization or use of Yttrium-90 micro-spheres remains unsolved.
All studies in our meta-analysis showed an increased survival in the groups treated with complete surgical resection of liver metastases, but none of the included studies was randomized (53-55).
Few studies have been designed to evaluate the impact of non-surgical therapy. Osborne et al. compared the difference between surgical therapy versus embolization and concluded that surgery is superior to embolization for symptom control and also found that palliative resection of liver metastases had a better survival than embolization (53).
Crucial questions remain unsolved. What kind of palliative treatment should be chosen facing non-resectable NETs liver metastases, in hopes to achieve prolonged outcomes and palliation? Which is the role of liver surgery in down-staging hepatic metastases extent allowing other non-surgical therapies to control the liver disease? How to accurately assess the full extent of metastatic disease in order to select the proper surgical management of patients with NET, particularly when considering transplant candidates?
Following full-text evaluation of 38 articles potentially eligible for this review, 35 of them did not described the outcome differences between curative hepatic resections and palliative surgery or did not reported any HR or comparison by Kaplan-Meier curves. Therefore a total amount of 169 patients affected by NET liver metastases, 81 treated by curative surgery and 88 by palliative surgery, were considered. In this meta-analysis, even if the number of studied cases is limited, curative resection of liver NETs metastases showed a non significant increased survival compared to palliative surgical resection HR 0.40 (95% CI: 0.14-1.11). Unfortunately, the results of our review does not allow definite answers but, taking into consideration this outcome and the concordant increased survival recorded in all patients treated with surgical resection of liver metastases (12), a curative and even palliative resection of neuroendocrine liver metastases should be considered in order to improve patient’s survival.
Conclusions
Liver metastases are frequently encountered in patients with NETs. For patients with resectable hepatic disease, the majority of the authors indicate liver resection, as this treatment results more likely to offer the best long-term outcome. All patients should be considered for curative surgical treatment but also palliative resection of liver metastases can be suggested. The advantage that can be potentially achieved with surgery, is that of removing all gross disease. Since there are no randomized clinical trials confirming clear advantages of hepatic resection, no definite conclusion on the impact of this aggressive approach can be achieved. However, basing on the results of this meta-analysis, palliative surgery, especially in patients with symptomatic disease, has a positive role (if at least 90% of the gross disease can be resected). In the future, new clinical and biological prognostic factors could be of help for the better identification of those patients who might benefit from hepatic surgical therapy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Gaujoux S, Gonen M, Tang L, et al. Synchronous resection of primary and liver metastases for neuroendocrine tumors. Ann Surg Oncol 2012;19:4270-7. [PubMed]
- Janson ET, Holmberg L, Stridsberg M, et al. Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol 1997;8:685-90. [PubMed]
- Hellman P, Lundström T, Ohrvall U, et al. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg 2002;26:991-7. [PubMed]
- Norton JA. Endocrine tumours of the gastrointestinal tract. Surgical treatment of neuroendocrine metastases. Best Pract Res Clin Gastroenterol 2005;19:577-83. [PubMed]
- Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol 2007;47:460-6. [PubMed]
- Pape UF, Jann H, Müller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer 2008;113:256-65. [PubMed]
- Pape UF, Berndt U, Müller-Nordhorn J, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer 2008;15:1083-97. [PubMed]
- Musunuru S, Chen H, Rajpal S, et al. Metastatic neuroendocrine hepatic tumors: resection improves survival. Arch Surg 2006;141:1000-4; discussion 1005. [PubMed]
- Landry CS, Scoggins CR, McMasters KM, et al. Management of hepatic metastasis of gastrointestinal carcinoid tumors. J Surg Oncol 2008;97:253-8. [PubMed]
- Frilling A, Sotiropoulos GC, Li J, et al. Multimodal management of neuroendocrine liver metastases. HPB (Oxford) 2010;12:361-79. [PubMed]
- Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg 2011;128:305-10. [PubMed]
- Bacchetti S, Bertozzi S, Londero AP, et al. Surgical treatment and survival in patients with liver metastases from neuroendocrine tumors: a meta-analysis of observational studies. Int J Hepatol 2013;2013:235040.
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6:e1000097. [PubMed]
- Norlén O, Stålberg P, Zedenius J, et al. Outcome after resection and radiofrequency ablation of liver metastases from small intestinal neuroendocrine tumours. Br J Surg 2013;100:1505-14. [PubMed]
- Taner T, Atwell TD, Zhang L, et al. Adjunctive radiofrequency ablation of metastatic neuroendocrine cancer to the liver complements surgical resection. HPB (Oxford) 2013;15:190-5. [PubMed]
- Saxena A, Chua TC, Chu F, et al. Optimizing the surgical effort in patients with advanced neuroendocrine neoplasm hepatic metastases: a critical analysis of 40 patients treated by hepatic resection and cryoablation. Am J Clin Oncol 2012;35:439-45. [PubMed]
- Saxena A, Chua TC, Zhao J, et al. Liver-directed therapy for neuroendocrine neoplasm hepatic metastasis prolongs survival following progression after initial surgery. J Surg Oncol 2012;105:342-50. [PubMed]
- Whitney R, Tatum C, Hahl M, et al. Safety of hepatic resection in metastatic disease to the liver after yttrium-90 therapy. J Surg Res 2011;166:236-40. [PubMed]
- Gedaly R, Daily MF, Davenport D, et al. Liver transplantation for the treatment of liver metastases from neuroendocrine tumors: an analysis of the UNOS database. Arch Surg 2011;146:953-8. [PubMed]
- Lillegard JB, Fisher JE, Mckenzie TJ, et al. Hepatic resection for the carcinoid syndrome in patients with severe carcinoid heart disease: does valve replacement permit safe hepatic resection? J Am Coll Surg 2011;213:130-6; discussion 136-8. [PubMed]
- Máthé Z, Tagkalos E, Paul A, et al. Liver transplantation for hepatic metastases of neuroendocrine pancreatic tumors: a survival-based analysis. Transplantation 2011;91:575-82. [PubMed]
- Saxena A, Chua TC, Sarkar A, et al. Progression and survival results after radical hepatic metastasectomy of indolent advanced neuroendocrine neoplasms (NENs) supports an aggressive surgical approach. Surgery 2011;149:209-20. [PubMed]
- Glazer ES, Tseng JF, Al-Refaie W, et al. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford) 2010;12:427-33. [PubMed]
- Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol 2010;17:3129-36. [PubMed]
- Ahmed A, Turner G, King B, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer 2009;16:885-94. [PubMed]
- Frilling A, Li J, Malamutmann E, et al. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br J Surg 2009;96:175-84. [PubMed]
- Chambers AJ, Pasieka JL, Dixon E, et al. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery 2008;144:645-51; discussion 651–3. [PubMed]
- Cho CS, Labow DM, Tang L, et al. Histologic grade is correlated with outcome after resection of hepatic neuroendocrine neoplasms. Cancer 2008;113:126-34. [PubMed]
- Eriksson J, Stålberg P, Nilsson A, et al. Surgery and radiofrequency ablation for treatment of liver metastases from midgut and foregut carcinoids and endocrine pancreatic tumors. World J Surg 2008;32:930-8. [PubMed]
- Kianmanesh R, Sauvanet A, Hentic O, et al. Two-step surgery for synchronous bilobar liver metastases from digestive endocrine tumors: a safe approach for radical resection. Ann Surg 2008;247:659-65. [PubMed]
- Le Treut YP, Grégoire E, Belghiti J, et al. Predictors of long-term survival after liver transplantation for metastatic endocrine tumors: an 85-case French multicentric report. Am J Transplant 2008;8:1205-13. [PubMed]
- Hibi T, Sano T, Sakamoto Y, et al. Surgery for hepatic neuroendocrine tumors: a single institutional experience in Japan. Jpn J Clin Oncol 2007;37:102-7. [PubMed]
- Jensen EH, Kvols L, McLoughlin JM, et al. Biomarkers predict outcomes following cytoreductive surgery for hepatic metastases from functional carcinoid tumors. Ann Surg Oncol 2007;14:780-5. [PubMed]
- Reddy SK, Barbas AS, Marroquin CE, et al. Resection of noncolorectal nonneuroendocrine liver metastases: a comparative analysis. J Am Coll Surg 2007;204:372-82. [PubMed]
- Sartori P, Mussi C, Angelini C, et al. Palliative management strategies of advanced gastrointestinal carcinoid neoplasms. Langenbecks Arch Surg 2005;390:391-6. [PubMed]
- Elias D, Lasser P, Ducreux M, et al. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: a 15-year single center prospective study. Surgery 2003;133:375-82. [PubMed]
- Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg 2003;197:29-37. [PubMed]
- Gulec SA, Mountcastle TS, Frey D, et al. Cytoreductive surgery in patients with advanced-stage carcinoid tumors. Am Surg 2002;68:667-71; discussion 671-2. [PubMed]
- Chung MH, Pisegna J, Spirt M, et al. Hepatic cytoreduction followed by a novel long-acting somatostatin analog: a paradigm for intractable neuroendocrine tumors metastatic to the liver. Surgery 2001;130:954-62. [PubMed]
- Coppa J, Pulvirenti A, Schiavo M, et al. Resection versus transplantation for liver metastases from neuroendocrine tumors. Transplant Proc 2001;33:1537-9. [PubMed]
- Nave H, Mössinger E, Feist H, et al. Surgery as primary treatment in patients with liver metastases from carcinoid tumors: a retrospective, unicentric study over 13 years. Surgery 2001;129:170-5. [PubMed]
- Yao KA, Talamonti MS, Nemcek A, et al. Indications and results of liver resection and hepatic chemoembolization for metastatic gastrointestinal neuroendocrine tumors. Surgery 2001;130:677-82; discussion 682-5. [PubMed]
- Grazi GL, Cescon M, Pierangeli F, et al. Highly aggressive policy of hepatic resections for neuroendocrine liver metastases. Hepatogastroenterology 2000;47:481-6. [PubMed]
- Chen H, Hardacre JM, Uzar A, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg 1998;187:88-92; discussion 92-3. [PubMed]
- Lehnert T. Liver transplantation for metastatic neuroendocrine carcinoma: an analysis of 103 patients. Transplantation 1998;66:1307-12. [PubMed]
- Dousset B, Saint-Marc O, Pitre J, et al. Metastatic endocrine tumors: medical treatment, surgical resection, or liver transplantation. World J Surg 1996;20:908-14; discussion 914-5. [PubMed]
- McEntee GP, Nagorney DM, Kvols LK, et al. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery 1990;108:1091-6. [PubMed]
- Osborne DA, Zervos EE, Strosberg J, et al. Improved outcome with cytoreduction versus embolization for symptomatic hepatic metastases of carcinoid and neuroendocrine tumors. Ann Surg Oncol 2006;13:572-81. [PubMed]
- Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000;190:432-45. [PubMed]
- Que FG, Nagorney DM, Batts KP, et al. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg 1995;169:36-42; discussion 42-3. [PubMed]
- Saxena A, Chua TC, Perera M, et al. Surgical resection of hepatic metastases from neuroendocrine neoplasms: a systematic review. Surg Oncol 2012;21:e131-141. [PubMed]
- Higgins JP, Green S, Collaboration C. eds. Cochrane handbook for systematic reviews of interventions, volume 5. Wiley Online Library, 2008.
- Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [PubMed]
- Schimmack S, Svejda B, Lawrence B, et al. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch Surg 2011;396:273-98. [PubMed]
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. [PubMed]
- Rorstad O. Prognostic indicators for carcinoid neuroendocrine tumors of the gastrointestinal tract. J Surg Oncol 2005;89:151-60. [PubMed]
- Soga J. The term “carcinoid” is a misnomer: the evidence based on local invasion. J Exp Clin Cancer Res 2009;28:15. [PubMed]
- Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39:707-12. [PubMed]
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:1-18. [PubMed]
- Turaga KK, Kvols LK. Recent progress in the understanding, diagnosis, and treatment of gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin 2011;61:113-32. [PubMed]
- Norton JA, Warren RS, Kelly MG, et al. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery 2003;134:1057-63; discussion 1063-5. [PubMed]
- Srirajaskanthan R, Toumpanakis C, Meyer T, et al. Review article: future therapies for management of metastatic gastroenteropancreatic neuroendocrine tumours. Aliment Pharmacol Ther 2009;29:1143-54. [PubMed]
- Eriksson B. New drugs in neuroendocrine tumors: rising of new therapeutic philosophies? Curr Opin Oncol 2010;22:381-6. [PubMed]
- Strosberg JR, Cheema A, Kvols LK. A review of systemic and liver-directed therapies for metastatic neuroendocrine tumors of the gastroenteropancreatic tract. Cancer Control 2011;18:127-37. [PubMed]
- Sutton R, Doran HE, Williams EM, et al. Surgery for midgut carcinoid. Endocr Relat Cancer 2003;10:469-81. [PubMed]
- Bonaccorsi-Riani E, Apestegui C, Jouret-Mourin A, et al. Liver transplantation and neuroendocrine tumors: lessons from a single centre experience and from the literature review. Transplant International 2010;23:668-78. [PubMed]
- Oberg K, Castellano D. Current knowledge on diagnosis and staging of neuroendocrine tumors. Cancer Metastasis Rev 2011;30 Suppl 1:3-7. [PubMed]
- Frilling A, Rogiers X, Malagó M, et al. Treatment of liver metastases in patients with neuroendocrine tumors. Langenbecks Arch Surg 1998;383:62-70. [PubMed]