The basics of ultrasound elastography for diagnosis, assessment, and staging breast cancer-related lymphedema: a systematic review of the literature
Introduction
Lymphedema is a chronic condition characterized by the accumulation of protein-rich liquid in the interstitial space of tissues caused by the inability of the lymphatics to transport lymph fluid back to the lymphatic system (1). Breast cancer-related lymphedema (BCRL) typically occurs in 20% to 94% of patients, usually between 2 to 5 years after surgery (2). The National Cancer Institute predicts that there will be almost 4 million breast cancer survivors by January 2024 (3), and as a result, the incidence of BCRL will increase. Potential causes related to BCRL include radiotherapy and lymph node biopsy or dissection (1,4). Diagnosis is mostly clinical (5), and it has been classified into four stages by the International Society of Lymphology based on clinical characteristics. Stages 0 and I correspond to the early accumulation of high protein fluid, and pitting may occur. Stages II and III involve fibrosis, fat deposits, and trophic skin changes, and pitting starts to disappear (6). Currently, there is no specific tool for diagnosis at early stages when symptoms have not appeared. However, attempts to stage and evaluate the disease objectively have been suggested. The most popular tests to characterize BCRL include the following: measurement of arm circumference; perometry, which assesses the volume of the affected arm compared with the non-affected arm; and bioimpedance, which scans resistance to painless electric currents passed through the arm (4). On the other hand, lymphoscintigraphy imaging technique is considered the criterion standard for diagnosis of BCRL, using a radiolabeled substance to visualize the lymphatic system and reveal the presence and caliber of lymphatic vessels, lymph nodes, collaterals, and delay in radionuclide uptake (7). However, this method is not usually preferred due to the lack a standard protocol, the invasiveness of the procedure, and the radiation exposure to the patient.
Other imaging methods used to evaluate BCRL are computed tomography, magnetic resonance imaging, and indocyanine green lymphography; however, they lack portability and are more expensive than the others (8). Ultrasonography is considered an easy and safe imaging test for evaluating thickness of skin and subcutaneous tissue, and as result, has been studied to assess lymphedema patients. In recent years, ultrasound elastography (UE) has been used to evaluate BCRL; however, parameters for assessing, diagnosing, and staging the disease have not been well established. In this systematic review, we aim to present the clinical studies to date to reveal the potential advantages and pitfalls regarding the use of UE in BCRL.
Methods
Study selection
Our systematic review included in vivo studies on use of UE for patients with lymphedema of the upper limbs. Studies were included if they were original articles focused on testing UE in patients with BCRL written in English. Reviews and systematic reviews were excluded, as were studies in which UE was not tested in vivo in BCRL and those not specifying results for BCRL.
Data sources and search strategy
This study followed the guidelines outlined in the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA). A comprehensive systematic review was conducted by one author (Maria T. Huayllani) on June 25, 2019, in the PubMed, EMBASE, Ovid Healthstar and Ovid Medline databases searching for articles reporting on the use of UE in patients with BCRL. The keywords for the search strategy were “elastography” AND “lymphedema” in titles or abstracts.
Studies were identified and uploaded into EndNote (Clarivate). Manuscripts were screened manually by the first author and selected according to the inclusion and exclusion criteria in a two-step process by two authors (Maria T. Huayllani, Daniel Boczar). First, studies were reviewed based on the title and abstract and duplicates were removed. Second, the full text of the selected studies was screened for final selection. If the first author doubted selecting an article, the second author reviewed the article according to the selection criteria and both reviewers came to a consensus for the final decision.
Data pooling and data analysis
Relevant data were extracted and pooled describing the author, year of publication, participants, type of ultrasound, method, biomarker used to measure the results, standard comparison tool, application of the new method, and results.
Risk of bias assessment
We used the Grade of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group recommendations to assess the risk of bias of the studies (Table 1). We found a 100% of studies with high risk of bias for the adequate sequence generation and 100% of studies with unclear risk of bias for other possible bias. As a consequence, there were some limitations to consider proper of the nature of the included studies corresponding to a moderate to low level of evidence.

Full table
Results
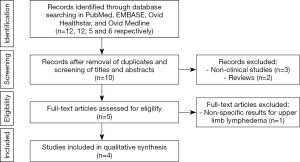
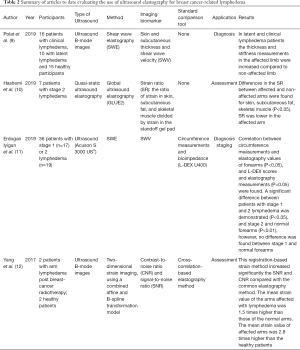
In our first search, 12, 12, 5 and 6 articles were found in total in PubMed, EMBASE, Ovid Healthstar, and Ovid Medline databases, respectively. From all these, only 4 studies met inclusion criteria (Figure 1). All included studies were published between 2017 and 2019. Detailed descriptions of the studies are provided in Table 2. UE methods tested included two-dimensional strain imaging (12), shear wave elastography (SWE) (11), and global UE (10). Different imaging biomarkers were used to compare the efficacy of each method, including skin and subcutaneous thickness (9), shear wave velocity (11), strain ratio (10), and contrast-to-noise ratio and signal-to-noise ratio (12). Two studies evaluated UE for assessment of patients with lymphedema, while one studied its use in diagnosis and staging of the disease (11) and another only in diagnosis of lymphedema (9). All studies found differences through UE evaluation between the affected and unaffected limb with lymphedema.
Full table
Discussion
Ultrasonography is a safe, easy, low-cost procedure for assessing patients with BCRL. Changes in BCRL include increasing thickness of the dermis, change from hypoechogenicity to hyperechogenicity of the subcutis, and fluid retention in the dermis, interlobular space, and superficial fascia. Although these changes may be hard to detect on ultrasound imaging (10), it can give a quantitative measure of the thickness of cutaneous, fascial, and surrounding tissue to assess BCRL (13). For instance, chronic lymphedema is characterized by a broadening of the subcutis with anechoic longitudinal columns and echogenic rims (14). Important limitations of ultrasound imaging include the lack of accuracy and difficulty differentiating BCRL from other causes of edema, such as cardiac, hepatic, or venous edema (14). Moreover, this technique is mainly subjective as it is operator-dependent and the pressure applied during the procedure may affect the results. To overcome these limitations, UE has been developed as an innovative and noninvasive technique based on the mechanical properties of tissue (15). This technique detects tissue stiffness as a response to a mechanical force like compression or shear wave (16). A softer tissue has more strain than a stiffer tissue when subjected to the same magnitude force (17). Two subtechniques are currently available, strain imaging and shear wave imaging. Strain imaging includes two approaches, strain elastography and acoustic radiation force impulse (ARFI) strain imaging (18). Strain elastography is operator-dependent, requiring manual compression, while in ARFI, the transducer is held steady and the tissue displacement is produced as an internal physiologic motion from an acoustic force generated by the UE system. As a consequence, magnitude of force is better controlled with ARFI, allowing evaluation of deeper organs (19,20). In both approaches, compression should be monitored and optimized to better contrast tissues. Shear wave imaging employs a dynamic stress that generates shear waves in parallel or perpendicular dimensions that have a velocity of propagating through the tissues that is usually tracked to provide a quantitative measure of tissue stiffness. The speed of the shear waves is directly proportional to the tissue stiffness (11). Different algorithms are applied to estimate mechanical parameter strain, or elasticity, and map this elasticity into an image (17).
In general, different methods have been suggested to be useful in patients with lymphedema. The results of strain elastography can be differentiated using different imaging biomarkers. Results are commonly reported as a subjective description of the color presentation in the elastogram, ranging from red (stiffer tissue) to blue (softer tissue) (21), as a semi-quantitative numeric scale score of the rate between the heterogeneity and distribution of colors in the elastogram (22), or as a strain ratio, which is the area of interest over the surrounding non-affected tissue within the same field of view (23). A limitation of common elastography is the potential for decorrelation noise from large or out-of-plane motions and non-rigid tissues deformations, as most tissues have a tissue motion and deformation in more than one dimension. Yan et al. (12) developed a two-dimensional strain technology to overcome this disadvantage and assessed feasibility of UE in 2 patients who developed arm lymphedema after radiotherapy due to breast cancer. They designed the device with a cuff attached to a manometer that generates low pressure to the arm without finding any trade-offs between resolution and noise level. Moreover, they observed a statistical difference in the strain values. The average strain values in the affected arms were 1.5 times higher than in normal arms (12). On the other hand, Erdogan Iyigun et al. (11) evaluated UE in the diagnosis and staging of lymphedema by using the SWE as an imaging biomarker in BCRL patients. They only considered patients with stage 1 and 2 of lymphedema, correlating their results with the circumference measurements and bioimpedance results. They found a significant difference between the elastography measurements using SWE between the normal and affected forearms. They also found a significant difference between patients with stage 1 and 2; however, when the shear wave velocity results were compared between affected and non-affected forearms by stage of disease, a significant difference was only identified in patients with stage 2 lymphedema (P<0.01). They also established that values of 1.78 or more of SWE would differentiate stage 2 from stage 1 lymphedema (11). Similarly, Polat et al. (9) assessed also the feasibility of UE by using SWE in patients with clinical lymphedema, latent lymphedema and healthy participants. They found a statistical difference in thickness and stiffness in the affected limbs of latent and clinical lymphedema compared with the non-affected arm (9). Hashemi et al. (10) proposed the use of an acoustic gel pad to produce the same pressure in both arms and increase the quality of the UE. They estimated determined strain values for the gel pad, skin, subcutaneous tissue, and skeletal muscle layers of affected and non-affected arms. Six different locations were measured in the patients’ arms. They found a strain ratio higher in the non-affected compared to the affected arm in all locations of subcutaneous fat tissue. They also found the specific locations to evaluate BCRL that had a higher difference of strain ratios, suggesting that the difference of mechanic properties of tissues is not limited to a single area (10).
These are important studies as they assess the applicability of UE as an early method of diagnosis, staging, and assessment of lymphedema in BCRL. However, these studies are limited by the lack of specific imaging biomarkers to compare results and the evaluation mechanical elasticity and stiffness alone, instead of tissue-fluid dynamics. For these reasons, more studies testing these UE methods, and eventually other imaging techniques, in greater sample sizes are necessary to establish the exact arm locations to evaluate, the best imaging biomarkers, and the specific cutoffs that may differentiate diagnosis and grade of disease.
Strengths and limitations
This is a systematic review of all English-language publications to date that probed the use of UE as a tool for assessing patients with BCRL in the English-language literature. We summarized the results of different methods of UE; therefore, the limitation of heterogeneity and possible biases proper of each study that we found through the risk of bias assessment should be considered. Another inherent limitation of reviews is the potential for search, selection, and publication biases. However, this systematic review is entirely descriptive, in alignment with the purpose of the study. To our knowledge, this is the first systematic review evaluating the use of ultrasound elastography for BCRL.
Conclusions
UE has shown efficacy in determining BCRL between affected and non-affected arms. Further studies to confirm its use in staging and diagnosis of early BCRL stages with greater sample sizes should be conducted.
Acknowledgments
Funding: This study was supported in part by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, by the Mayo Clinic Center for Individualized Medicine, and by the Plastic Surgery Foundation.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Xiaona Lu, Antonio Jorge Forte) for the series “Lymphedema” published in Gland Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs.2020.02.08). The series “Lymphedema” was commissioned by the editorial office without any funding or sponsorship. XL serves as the unpaid editorial board member of Gland Surgery from Aug 2019 to Jul 2021 and served as the unpaid Guest Editor of the series. AJF served as the unpaid Guest Editor of the series. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taghian NR, Miller CL, Jammallo LS, et al. Lymphedema following breast cancer treatment and impact on quality of life: a review. Crit Rev Oncol Hematol 2014;92:227-34. [Crossref] [PubMed]
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500-15. [Crossref] [PubMed]
- Shaitelman SF, Cromwell KD, Rasmussen JC, et al. Recent progress in the treatment and prevention of cancer-related lymphedema. CA Cancer J Clin 2015;65:55-81. [Crossref] [PubMed]
- Tandra P, Kallam A, Krishnamurthy J. Identification and Management of Lymphedema in Patients With Breast Cancer. J Oncol Pract 2019;15:255-62. [Crossref] [PubMed]
- Hayes SC, Janda M, Cornish B, et al. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J Clin Oncol 2008;26:3536-42. [Crossref] [PubMed]
- Warren AG, Brorson H, Borud LJ, et al. Lymphedema: a comprehensive review. Ann Plast Surg 2007;59:464-72. [Crossref] [PubMed]
- Brahma B, Yamamoto T. Breast cancer treatment-related lymphedema (BCRL): An overview of the literature and updates in microsurgery reconstructions. Eur J Surg Oncol 2019;45:1138-45. [Crossref] [PubMed]
- Michelotti A, Invernizzi M, Lopez G, et al. Tackling the diversity of breast cancer related lymphedema: Perspectives on diagnosis, risk assessment, and clinical management. Breast 2019;44:15-23. [Crossref] [PubMed]
- Polat AV, Ozturk M, Polat AK, et al. Efficacy of Ultrasound and Shear Wave Elastography for the Diagnosis of Breast Cancer-Related Lymphedema. J Ultrasound Med 2020;39:795-803. [Crossref] [PubMed]
- Hashemi HS, Fallone S, Boily M, et al. Assessment of Mechanical Properties of Tissue in Breast Cancer-Related Lymphedema Using Ultrasound Elastography. IEEE Trans Ultrason Ferroelectr Freq Control 2019;66:541-50. [Crossref] [PubMed]
- Erdogan Iyigun Z, Agacayak F, Ilgun AS, et al. The Role of Elastography in Diagnosis and Staging of Breast Cancer-Related Lymphedema. Lymphat Res Biol 2019;17:334-9. [Crossref] [PubMed]
- Yang X, Torres M, Kirkpatrick S, et al. Ultrasound 2D strain measurement for arm lymphedema using deformable registration: A feasibility study. PLoS One 2017;12:e0181250. [Crossref] [PubMed]
- Jeon Y, Beom J, Ahn S, et al. Ultrasonographic Evaluation of Breast Cancer-related Lymphedema. J Vis Exp 2017. [Crossref] [PubMed]
- Keo HH, Gretener SB, Staub D. Clinical and diagnostic aspects of lymphedema. Vasa 2017;46:255-61. [Crossref] [PubMed]
- Yang X, Torres M, Kirkpatrick S, et al. Ultrasound 2D Strain Estimator Based on Image Registration for Ultrasound Elastography. Proc SPIE Int Soc Opt Eng 2014. pii: 904018.
- Sigrist RMS, Liau J, Kaffas AE, et al. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017;7:1303-29. [Crossref] [PubMed]
- DeJong HM, Abbott S, Zelesco M, et al. The validity and reliability of using ultrasound elastography to measure cutaneous stiffness, a systematic review. Int J Burns Trauma 2017;7:124-41. [PubMed]
- Ophir J, Cespedes I, Ponnekanti H, et al. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging 1991;13:111-34. [Crossref] [PubMed]
- Gennisson JL, Deffieux T, Fink M, et al. Ultrasound elastography: principles and techniques. Diagn Interv Imaging 2013;94:487-95. [Crossref] [PubMed]
- Li GY, Cao Y. Mechanics of ultrasound elastography. Proc Math Phys Eng Sci 2017;473:20160841. [Crossref] [PubMed]
- Gaspari R, Blehar D, Mendoza M, et al. Use of ultrasound elastography for skin and subcutaneous abscesses. J Ultrasound Med 2009;28:855-60. [Crossref] [PubMed]
- Stachs A, Hartmann S, Stubert J, et al. Differentiating between malignant and benign breast masses: factors limiting sonoelastographic strain ratio. Ultraschall Med 2013;34:131-6. [PubMed]
- Suehiro K, Morikage N, Murakami M, et al. Skin and subcutaneous tissue strain in legs with lymphedema and lipodermatosclerosis. Ultrasound Med Biol 2015;41:1577-83. [Crossref] [PubMed]