
A new mouse model of poorly differentiated thyroid carcinoma and its implications for human disease
Among the primary malignancies of the thyroid gland, the vast majority is represented by differentiated thyroid carcinoma (DTC), which comprises papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC) and oncocytic or Hürthle cell thyroid carcinoma (HTC). In most cases, these carcinomas are of a rather indolent nature and are treated very effectively with surgery, followed by radioactive iodine (RAI) in selected cases. Most of the disease-related mortality among patients with DTC concerns tumors that are RAI-refractory, which are rare; thus, the overall mortality associated with DTC is quite low. At the other end of the spectrum of primary thyroid malignancies lies anaplastic thyroid carcinoma (ATC), an aggressive and rapidly progressive cancer with a very high mortality rate. Because ATC does not express markers of thyroid differentiation, it is also termed undifferentiated thyroid carcinoma (UTC). This lack/loss of differentiation has important therapeutic and prognostic implications: because ATC cells do not express functional sodium-iodide symporter (NIS), RAI treatment has no place in the management of these tumors.
Between these two extremes, DTC and ATC, lies a group of tumors classified as poorly differentiated thyroid carcinoma (PDTC). These tumors do not meet the diagnostic criteria of ATC because they retain features of thyroid differentiation, albeit to varying degrees. Despite this variability, the histological diagnosis of PDTC does not pose problems, as its recognition is based on the so-called Turin criteria (1), which have been endorsed also by the World Health Organization classification of tumors of endocrine organs (2). Briefly, PDTC is defined as a thyroid malignant neoplasm (with extensive capsular and vascular invasion) that shows necrosis and a high mitotic activity. PDTC and DTC can coexist in the same patient, which is important to recognize because studies have shown that a PDTC component >10% in an otherwise DTC can have a negative impact on prognosis (3). PDTC can also be a precursor of ATC, and the two tumor types can also coexist in the same patient and they can have various molecular alterations in common (4).
In terms of its clinical behavior, PDTC is often, but not always, an aggressive tumor, including being RAI-refractory; thus, the overall prognosis of PDTC is intermediate between the favorable prognosis of DTC and the pessimistic prognosis of ATC. However, and in contrast to ATC that warrants immediate aggressive treatment, it is generally not possible to predict in an individual patient with PDTC, at the moment of initial diagnosis, whether the tumor will behave aggressively or whether it can be cured by surgery and RAI. Thus, the initial treatment of most patients with PDTC is similar to that of “high-risk” patients with DTC (i.e., total thyroidectomy and RAI); this is sufficient for many patients, yet for others it may represent a loss of precious time. Therefore, further research is warranted to understand the pathogenesis and tumor evolution of PDTC, including the mechanisms involved in the maintenance or loss of differentiated features (e.g., NIS expression and functionality) and their clinical (e.g., RAI uptake) and prognostic correlates (RAI-sensitive vs. RAI-refractory nature).
A recent study by the group of Yuri Nikiforov published in the October 2019 issue of Thyroid aimed to address some of these questions using a mouse model of PDTC (5). In previous work, the same group had created mice with targeted overexpression in thyroid follicular cells [using a promoter of the gene encoding thyroglobulin (Tg)] of a STRN-ALK fusion protein, and had shown that these mice develop tumors that meet the histological diagnostic criteria of PDTC (6). ALK (anaplastic lymphoma kinase) is a receptor tyrosine kinase expressed in various tissues; the ALK gene is a known oncogene that is activated by chromosomal translocations generating ALK fusion genes. Among the various early driver mutations encountered in thyroid carcinomas (primarily BRAF and RAS point mutations), ALK fusions are found in small subsets of PTC (1.6%), PDTC (9%) and ATC (4%) (7). In thyroid carcinomas, the most frequent translocation partner is the STRN gene that encodes striatin, a calmodulin-dependent scaffolding protein (8). STRN-ALK fusion proteins lead to constitutive activation of ALK signaling via dimerization mediated by the coiled-coil domain of striatin; this induces kinase-dependent, thyroid-stimulating hormone (TSH)-independent proliferation of thyroid cells (7).
Within a thyroid tissue proliferating under the influence of an early genetic driver event, the occurrence of late genetic driver events, such as loss of the anti-oncogene p53 (the so-called “guardian of the genome”) (9), can trigger a rapid and more aggressive carcinogenic process that can culminate in PDTC and/or ATC (3). Therefore, to better account for the contribution of late genetic driver events, in the present study Nikitski et al. expanded on their previously characterized STRN-ALK fusion-driven PDTC model by deleting the p53 gene (via Cre-recombinase also expressed by the same Tg promoter) specifically in the same thyroid follicular cells that overexpressed the STRN-ALK fusion transgene (5). A subset of ALK-overexpressing, homozygous p53-deleted mice were also treated with antithyroid medications (thyroid hormone synthesis inhibitors and perchlorate) to increase the serum levels of TSH (considered to promote thyroid cell growth and proliferation) and thus test the effect that this further stimulation might have on tumor development.
Using a special micro-instrument, the mice were monitored non-invasively by thyroid ultrasound for the development of thyroid tumors, which appeared from the age of 7 months on normal diet or 5 months under goitrogen treatment. Some of the tumors that were followed by ultrasound in these mice started to grow more rapidly; after sacrifice of the animals, these were shown to be foci of PDTC growing next to foci of PTC, supporting the concept that PDTC can result from the evolution and dedifferentiation of PTC. Indeed, these mice developed PTC (most frequently) and PDTC but also ATC (less frequently). Different types of thyroid carcinoma could be observed in the same mouse, the most frequent being the combination of PTC and PDTC. The most dominant type was PDTC, occupying the larger part of the thyroid parenchyma. In mice treated with goitrogen, not only was the time to tumor development shorter, but the frequency of tumor development was also higher and the distribution of the observed tumor types was shifted towards dedifferentiation. Irrespective of goitrogen treatment, the tumors observed showed aggressive features such as extrathyroidal extension, invasion of trachea and blood vessels as well as frequent lung metastases.
Histologically, the PDTC tumors developed in these mice fulfilled the aforementioned Turin diagnostic criteria. However, an interesting observation was that these PDTC tumors could be classified into two histological subtypes according to cell morphology: The PDTC1 subtype comprised cells with a substantial amount of cytoplasm and small nuclei with dense, evenly distributed chromatin (Figure 1); these features generally resemble those of DTC cells. On the other hand, PDTC2 tumors comprised cells with less cytoplasm and larger, vesicular nuclei that had prominent and often multiple nucleoli (Figure 1); these features bear no resemblance to those of DTC cells. Most mice developed either PDTC1 or PDTC2 tumors, whereas some mice developed both PDTC1 and PDTC2 tumors, located next to each other; in those cases, the correlation between the ultrasonographic follow-up of the mice and the histopathology results showed that PDTC1 tumors developed earlier than PDTC2 tumors in the same mouse.
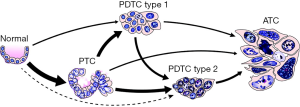
The two types of PDTC distinguished based on morphology turned out to be distinct also in terms of the expression of genes related to follicular thyroid cell differentiation. Specifically, the expression of thyroid-specific genes was analyzed by RNA sequencing and the abundance of thyroid-specific proteins was studied by immunohistochemistry. These analyses showed that PDTC1 tumors retained immunoreactivity of thyroid differentiation markers like Tg and of general epithelial markers like E-cadherin, whereas these proteins were undetectable in PDTC2 tumors. Similarly, at the mRNA level, PDTC1 tumors showed higher expression levels of the thyroid-specific genes Tg, Nis, thyroid peroxidase (Tpo) and dual-oxidase 2 (Duox2) as compared to PDTC2 tumors.
Based on these novel findings, the authors draw interesting conclusions regarding the pathogenesis of PDTC associated with STRN-ALK fusion and p53 loss. The model shown in Figure 1 summarizes the putative tumorigenesis, progression and dedifferentiation process in these mice, with the main pathway being from PTC to PDTC, to then possibly also ATC. Another important conclusion is that two distinct types of PDTC can develop, with PDTC2 being more advanced and less differentiated, both in terms of cell morphology and in terms of thyroid-specific gene expression. Both types can derive from PTC, or, less likely, from normal thyroid tissue; progression from PDTC1 to PDTC2 is possible, and both types may also progress to ATC (Figure 1).
The authors also speculate that the existence of two subtypes of PDTC, a less aggressive one that retains differentiation markers and a more aggressive one that has lost them, might be related to the corresponding variable clinical behavior of PDTC in humans. For example, approximately half of PDTC tumors in patients retain the ability to concentrate RAI (10). It might thus be that these tumors are similar, in terms of histology and gene expression, to the PDTC1 tumors observed in mice in the present study, whereas those that are RAI-refractory might be similar to PDTC2 tumors. However, it is currently unknown whether distinct subtypes of PDTC exist in humans, and this issue certainly warrants clarification in further studies. Also, as the authors acknowledge, it will be important to test whether the higher Nis expression observed in PDTC1 tumors in mice correlates with ability to concentrate RAI, as this question was not addressed in the present study (5).
This elegant study has several strengths, including the genetic introduction of clinically-relevant mutations, the non-invasive monitoring of the animals, the investigation of the contribution of goitrogen, and the detailed characterization of tumor types within and between animals, among others. On the other hand, it also has some limitations that merit consideration. One such limitation, acknowledged by the authors, is the use of a Tg promoter to drive the expression of the STRN-ALK fusion transgene as well as to trigger the recombination events deleting p53. Because Tg expression decreases with tumor dedifferentiation, this can have confounding effects on the degree of STRN-ALK expression and its associated phenotypes. For example, it may explain the relatively low penetrance of ATC in these mice. It also makes these mice unsuitable as a model to test the therapeutic effects of ALK inhibitors. The authors state that they plan to address this issue by using a doxycycline-inducible promoter in the place of the Tg promoter (5).
There are some additional limitations that are not discussed by the authors but may have clinical implications: (I) the model combined an early genetic event in PDTC evolution (i.e., the STRN-ALK fusion) with a late genetic event (loss of p53), and both of these events were triggered simultaneously and from early developmental stages using the Tg promoter. Thus, the stepwise process of tumor development with sequential early and late events was not recapitulated in a strict manner. However, this is understandable for the present study, given the complexity of genetic and pharmacological manipulations that would be necessary to mimic more closely the sequence and timing of human PDTC-relevant events; (II) also with reference to the genetic events modeled, as mentioned above, ALK fusions are found only in a minority of PTC, PDTC and ATC in humans (7). Thus, the clinical relevance of the findings for the vast majority of PDTC that do not harbor ALK fusions remains unknown. In that regard, the envisioned clinicopathological studies to investigate whether PDTC subtypes corresponding to PDTC1 and PDTC2 exist in humans should ideally consider the specific mutations that the respective tumors harbor; (III) further regarding tumor genetic composition, it is also important to note that the present study did not investigate the presence of any additional mutations acquired by the PDTC tumors that might account for the different cellular behaviors and evolution paths between PDTC1 and PDTC2 (Figure 1). Since loss of p53 is well-known to lead to genome instability, secondary mutational events are expected to accumulate during tumor progression, and it would be very interesting to map such events by comparing the genetic composition of PTC, PDTC1, PDTC2 and ATC tumors in these animals. It is possible that the authors, who are renowned experts in the genetics of thyroid carcinoma, may be currently working to address this question; (IV) lastly, the authors do not discuss how the possible existence of two distinct types of PDTC with different cell morphology may facilitate, or rather, further complicate, the preoperative diagnosis of PDTC by fine-needle aspiration biopsy, which remains a major challenge (11).
In conclusion, the elegant study by Nikitski et al. yields new insights into the pathogenesis of PDTC in a genetically defined mouse model (5). It represents a logical extension of a previous study by the same group (6), and it is expected that it will be followed by mouse and human clinicopathological studies to gain further insights with potential implications for the corresponding human disease.
Acknowledgments
Funding: GP Sykiotis is funded by Swiss National Science Foundation projects 31003A_182105 and IZCOZ0_177070 and by Leenaards Foundation 2016 Fellowship for Academic Promotion in Clinical Medicine.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs.2020.01.02). MB is an employee of Synlab Pathology. GPS has received honoraria from IBSA; unrestricted research grants from IBSA, Merck and AlfaSigma; and travel grants from Sanofi-Genzyme.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Volante M, Collini P, Nikiforov YE, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol 2007;31:1256-64. [Crossref] [PubMed]
- WHO Classification of Tumours of Endocrine Organs. Lyon: IARC; 2017.
- Dettmer MS, Schmitt A, Komminoth P, et al. Poorly differentiated thyroid carcinoma: An underdiagnosed entity. Pathologe 2019. [Epub ahead of print]. [Crossref]
- Xu B, Ghossein R. Genomic Landscape of poorly Differentiated and Anaplastic Thyroid Carcinoma. Endocr Pathol 2016;27:205-12. [Crossref] [PubMed]
- Nikitski AV, Rominski SL, Condello V, et al. Mouse Model of Thyroid Cancer Progression and Dedifferentiation Driven by STRN-ALK Expression and Loss of p53: Evidence for the Existence of Two Types of Poorly Differentiated Carcinoma. Thyroid 2019;29:1425-37. [Crossref] [PubMed]
- Nikitski AV, Rominski SL, Wankhede M, et al. Mouse Model of Poorly Differentiated Thyroid Carcinoma Driven by STRN-ALK Fusion. Am J Pathol 2018;188:2653-61. [Crossref] [PubMed]
- Kelly LM, Barila G, Liu P, et al. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci U S A 2014;111:4233-8. [Crossref] [PubMed]
- Gaillard S, Bartoli M, Castets F, et al. Striatin, a calmodulin-dependent scaffolding protein, directly binds caveolin-1. FEBS Lett 2001;508:49-52. [Crossref] [PubMed]
- Lane DP. Cancer. p53, guardian of the genome. Nature 1992;358:15-6. [Crossref] [PubMed]
- de la Fouchardière C, Decaussin-Petrucci M, Berthiller J, et al. Predictive factors of outcome in poorly differentiated thyroid carcinomas. Eur J Cancer 2018;92:40-7. [Crossref] [PubMed]
- Saglietti C, Onenerk AM, Faquin WC, et al. FNA diagnosis of poorly differentiated thyroid carcinoma. A review of the recent literature. Cytopathology 2017;28:467-74. [Crossref] [PubMed]


