
Adrenal incidentaloma: cardiovascular and metabolic effects of mild cortisol excess
Introduction
An adrenal incidentaloma (AI) is a lesion that is discovered during imaging of the abdomen for indications not associated with evaluation of the adrenal gland (1,2). Incidence has increased with more frequent use of axial imaging modalities such as computed tomography (CT) and magnetic resonance imaging (1). The overall prevalence is 4.2%, increasing with age (3) with around 10% of patients over 70 having an AI on CT (1,4). AI can further be categorized by assessment of the hypothalamic-pituitary-adrenal (HPA) axis (4,5). In general, and depending on the criteria used, the majority (70%) of AI are classified as non-functioning, with adrenal cortisol secretion causing mild cortisol excess (MCE) in 15–30% (6). In these latter patients, typical clinical features of hypercortisolism are absent with the condition previously being termed “subclinical Cushing’s syndrome (CS)” (1). In this review we avoid the term subclinical CS and instead we use MCE, defined as mild hypercortisolism with AI but in the absence of classical clinical features of CS (7).
What defines MCE is debated. A variety of cut-offs are used to define MCE when using serum cortisol values after dexamethasone testing or salivary or urinary cortisol values. Strengths and limitations of the biochemical assays and cut-offs used in the literature have already been reviewed comprehensively (8,9).
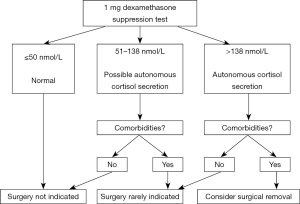
Recent European Society of Endocrinology (ESE) guidelines aimed to clarify this issue and suggested all patients with AI undergo a 1 mg overnight dexamethasone suppression test (ONDST) (4). In the ESE guidelines, a serum cortisol ≤50 nmol/L (1.8 µg/dL) post 1 mg ONDST is regarded as excluding hypercortisolism, values between 51 to 138 nmol/L are referred to as “possible autonomous cortisol secretion (ACS)”, and values >138 nmol/L termed “ACS” (Figure 1). These guidelines, however, emphasize that post ONDST serum cortisol values should not be interpreted as absolute arbitrary cut-offs. Rather, MCE in AI is best conceptualized as a biochemical continuum of excess cortisol. Thus, when formulating a management strategy, the patients age, past medical history, co-morbidities and biochemistry all need to be considered holistically.
Progression of AI to CS is rare with a recent meta-analysis finding only 0.2% of patients with MCE developed CS and none of the adenomas underwent transformation to adrenocortical carcinoma (10).
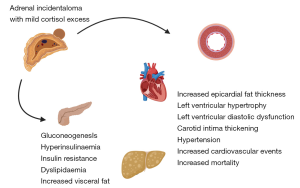
Several retrospective studies have suggested increased prevalence of cardiovascular and metabolic risk factors associated with MCE including hypertension, increased left ventricular mass, impaired glucose tolerance, increased visceral fat and mortality (11-17) (Figure 2). Despite increased morbidity and mortality, the majority of patients with AI do not receive the recommended initial hormone investigations (18,19).
The management of AI is another area with inconsistencies, with some studies demonstrating that early surgical intervention leads to improvement of cardiovascular morbidities and others showing no significant difference post adrenalectomy (20-24).
In this review, we aim to provide an overview of the cardiovascular and metabolic morbidities associated with MCE in the context of AI and briefly discuss management in light of the best available evidence.
Detection of MCE
AI are commonly found on routine imaging done for other causes. Briefly, characteristics of a benign lesion on non-contrast CT would include a mass with regular and smooth margins, homogenous in texture and Hounsfield units ≤10 (1,4).
As supported by the recent ESE guidelines, it is recommended that all patients with an AI noted on CT should be carefully assessed for evidence of hormonal excess. In addition to a 1 mg ONDST as described, initial investigations should include a basal morning plasma adrenocorticotrophic hormone (ACTH) measurement. In addition, in some circumstances, additional evaluation using a 24-h urinary free cortisol (UFC) collection and/or late-night salivary cortisol assay and repetition of the dexamethasone test in 3–12 months maybe needed (4).
Cardiovascular effects of MCE
Structural changes
In overt CS cardiovascular disease is a common cause of morbidity and mortality (25,26). Cushing’s disease has been shown to be associated with cardiac changes including left ventricular and interventricular septal hypertrophy on echocardiography (27-29), and more recently on cardiac magnetic resonance imaging (29), with increases in left ventricular mass being reversible and left ventricular systolic function improved following treatment of the underlying disorder.
Another group performed echocardiography on 46 patients with AI and 30 healthy controls (17). Within the AI group, 40 patients had a non-functioning adenoma (NFAI) and 6 “mild CS” as defined by 2 out of the following 3: 24-h UFC >193 nmol/day; serum free cortisol levels after 1 mg ONDST >138 nmol/L; and 08:00 ACTH levels <10 pg/mL. An increase in epicardial fat thickness was seen in the incidentaloma group (NFAI and mild CS) compared with controls (7.9±0.8 vs. 7.4±0.6 mm; P<0.01), with no significant difference between NFAI and mild CS groups. The lack of difference between those classified as NFAI and CS in this series may be due to the fact that those deemed to be NFAI by what are high cut-offs, actually had sufficient cortisol secretion to cause the changes. Left ventricular mass was also increased in the incidentaloma group (P<0.05) compared to controls, and also increased in the mild CS group compared to the NFAI group (P<0.01). These data support the concept of a continuum of effect with higher cortisol secretion causing more harm. This study was, however, limited by a small sample size. In addition, baseline characteristics show there was increased obesity and waist circumference in the AI group, possibly confounding results (17).
Similar results were obtained in the ERGO trial (Endocrine Cardiomyopathy in Cushing Syndrome: Response to Cyclic GMP PDE5 inhibitOrs), where a subset of 34 patients with AI and possible ACS, as defined by the ESE guidelines, and 37 with NFAI were analyzed (30). Significantly increased left ventricular hypertrophy was found in the ACS group compared with NFA (53% vs. 13.5%; P=0.001) and increased left ventricular mass index (LVMI) before and after adjusting for known contributors or LVMI normalized to body surface area. It was noted that LVMI was positively correlated to post ONDST serum cortisol levels. Other significant results included increased interventricular septal thickness and left ventricular diastolic dysfunction (LVDD) (82.3% vs. 35.1%; P=0.001), increased pulse wave velocity and reduced reflected wave transit time as a measure of arterial stiffness in the ACS group. Severity of LVDD was worse in the ACS group (grade 2: 35.3% vs. 8.1%; grade 3: 8.8% vs. 0%) (30). Pulse wave velocity is a marker of arterial stiffness and has been shown to be an independent predictor for both coronary heart disease and stroke in a healthy population (31) whilst moderate to severe LVDD is an independent predictor of mortality (32). Unfortunately, there was no age matched healthy control group with which to compare these findings.
Another study examined structural changes and compared 81 patients with AI (76 NFAI, 5 MCE) with 33 healthy subjects (33). MCE was diagnosed as serum cortisol levels >50 nmol/L post ONDST and with an ACTH value <10 pg/mL. On echocardiography, an increased thickness of the interventricular septum, posterior wall and carotid intima media in those with incidentalomas compared with healthy controls was reported. There was no difference found between NFAI and MCE groups, however, between group analyses were limited by the small sample size (33). Carotid intima thickening has been shown to be increased in AI in other studies (34,35) although it is unclear whether this is due to the direct effects of cortisol or an insulin resistant state.
Cardiovascular events (CVEs)
Hypertension is more prevalent in overt CS, alongside other cardiovascular risk factors (25). Several retrospective studies have also found increased prevalence of hypertension in patients with AI (4).
Morelli et al. analyzed retrospectively the records of 206 patients with AI (11). They defined MCE as post ONDST serum cortisol >138 nmol/L or the combination of 2 out of 3 of the following: ACTH <10 pg/mL, increased UFC, and post ONDST serum cortisol level >83 nmol/L. Prevalence of hypertension significantly increased in the MCE group at mean follow-up (82.5±32 months). The annual rate of CVE, defined as coronary heart disease or ischemic/hemorrhagic stroke, was increased in the MCE group (3.1% vs. 1.2%; P=0.004), although new CVE in those with no history of CVE at baseline was not increased. Given the higher cortisol level cut off, it is possible that those with lower levels of cortisol (>50 nmol/L) contributed to CVE, underestimating numbers. There was no comparison with healthy controls. At the same time this study only reviewed conservatively managed patients, excluding those who underwent adrenalectomy. Therefore, the results may again be underestimating the cardiovascular risk as those thought to have been at the highest risk of CVE may have been selected for surgery (11).
Di Dalmazi et al. performed a cross sectional study of 348 patients with AI (15). Patients were classified into the following groups based on post-dexamethasone serum cortisol: <50 nmol/L non-secretory; 50–138 nmol/L intermediate group; >138 nmol/L ACS group. In the intermediate group, patients with either high UFC or plasma ACTH <10 pg/mL were classified as intermediate major phenotype and the others as intermediate minor phenotype. Coronary heart disease was associated with both the MCE group (odds ratio 6.10; 95% CI: 1.41–26.49) and the intermediate phenotype groups (odds ratio 4.09; 95% CI: 1.47–11.38) compared with the non-secretory group, independent of other risk factors. The relatively small number in the ACS group, however, is possibly not representative of the wider population (15).
A further multicenter study was published in 2017 by Morelli et al. reviewing CVE during the 10 years prior to the diagnosis of AI and in the resulting follow-up period (mean 161.8±45.1 months) of their cohort of 518 patients (36). These results were then analyzed alongside post ONDST serum cortisol values showing CVE occurrence to be independently associated with cortisol levels as a continuous variable. Patients’ results were then grouped based on post ONDST serum cortisol: <50, 50–138 and >138 nmol/L with prevalence of CVE increasing, respectively (10.8%, 21.7%, 35.6%) and a 2.5-fold increase in CVE occurrence if post-dexamethasone cortisol was >50 nmol/L regardless of age, smoking habit, type 2 diabetes (T2D), hypertension or dyslipidemia. For every 28 nmol/L increase in post ONDST cortisol level, CVE prevalence increased by 1.3-fold. Patients with a post-ONDST serum cortisol >50 nmol/L had significantly elevated blood pressure compared with those with a cortisol <50 nmol/L (P=0.001). Being cross sectional, neither this study nor those discussed previously provide data on time exposure of cortisol (36).
Recently, Chiodini et al. in a cross-sectional study examined the relationship between serum cortisol levels post 1 mg ONDST and systemic hypertension in 216 eucortisolaemic post-menopausal women (37). Hypertension defined as a blood pressure >140/90 mmHg and/or need for anti-hypertensive treatment positively correlated with higher serum cortisol post 1 mg ONDST adjusting for age, body mass index (BMI) and T2D. Furthermore, cardiometabolic co-morbidities increased progressively with serum cortisol levels post 1 mg ONDST >30 nmol/L suggesting a threshold effect. However, 64.6% of those with hypertension had T2D and this is known to activate the HPA axis (38). Thus, whilst there may be a possible causative role for cortisol excess in driving hypertension and other co-morbidities in eucortisolaemic patients, these data warrant interpretation in the context of a high prevalence of T2D in this cohort (37).
Mortality
Di Dalmazi et al. reported increased mortality in patients with MCE (14). In a 15-year retrospective cohort study assessing 198 patients they found all-cause mortality 43% in the MCE group defined as a serum cortisol >50 nmol/L post 1 mg ONDST compared with 8.8% in the NFAI group (P=0.005). The main cause of mortality was cardiovascular disease (14). Furthermore, unadjusted survival for cardiovascular specific mortality was lower in those with MCE compared to those with NFAI (78.4% vs. 97.5%; P=0.02). Conversely, another group noted malignancy as the main cause of mortality in their cohort of MCE patients (39).
Our own group analyzed 206 patients with AI in a single center longitudinal cohort study with a mean follow-up of 4.2±2.3 years (13). Patients with a post ONDST serum cortisol level <50 nmol/L were considered to have a NFAI. Those with >50 nmol/L were classified into 2 groups: group 1 50–137 nmol/L (n=92) and group 2 >138 nmol/L (n=19). Correlation was found between post serum ONDST serum cortisol, adenoma size and also age. Compared with the <50 nmol/L group, hazard ratio for death in group 1 was 12 (95% CI: 1.6–92.6) and group 2 was 22 (95% CI: 2.6–188.3) with a significant worsening of survival rate with increasing post-dexamethasone cortisol. Crucially, in this study, cause of death was ascertained by death certification data. Of the 18 deaths, 50% were due to a circulatory cause and 33% attributed to respiratory/infective etiologies, both increased compared to general population data, and plausibly related to the biological effects of cortisol. In this study, patients with other causes of hypercortisolism such as depression or excess alcohol intake were not excluded which could have been falsely attributed to adenoma, although increased cortisol remained as a “marker for mortality”. Age at diagnosis was also significantly greater in the group 2 (13).
A recent multicenter cohort study analyzed the computerized tomography scans of 42,575 patients over a 4-year period (19). Of these, 969 were confirmed to have an AI and matched with 2,907 patients with no adenoma. A significant increase in mortality was reported in the patients with AI (36.4%) compared with those without adrenal nodules (31.6%, P=0.005; hazard ratio 1.19; 95% CI: 1.05–1.36). All-cause mortality was associated with an increased median nodule size (1.7 vs. 1.6 cm; P=0.03). There was also significantly increased diabetes, heart failure and malignancy in the incidentaloma group (19). These findings suggest that regardless of secretory levels of cortisol, which were not measured, the presence of an AI is associated with worse prognosis. Interestingly, within 12 months of detection of the incidentaloma, only 2.8% had at least one biochemical test to assess tumor function possibly suggesting a lack of awareness in the general medical community of initial investigation or maybe the association of “incidentaloma” with benign disease.
Metabolic effects of MCE
The relationship between MCE and metabolic dysfunction is complex and driven through a number of putative mechanisms that result in the metabolic syndrome characterized by: hyperinsulinemia, visceral obesity and dyslipidemia.
Hyperinsulinemia
MCE in the context of AI has been associated with a higher prevalence of impaired glucose tolerance and T2D in numerous studies (11,14,15,40). In a recent retrospective study of 242 patients with AI followed up for a mean duration of 7 years, the risk of developing diabetes in the AI cohort was significantly higher compared to incident risk of diabetes in controls without adrenal lesions (absolute risk of diabetes with AI =15.6%) (41). In this cohort, the prevalence of T2D rose linearly in those with a post ONDST cortisol >30 nmol/L (41).
Hyperinsulinemia and insulin resistance are important in the pathogenesis of T2D. A small observational study examining 13 patients was one of the first to report an association between AI and insulin resistance (42). The authors demonstrated hyperinsulinemia by showing elevated fasting insulin concentrations and elevated insulin concentrations following a 75-g oral glucose tolerance test (OGTT). In addition to a small sample size, the investigators did not report data on serum cortisol post ONDST in this cohort so the extent of cortisol excess if any is not known. Terzolo et al. conducted one of the first assessments of the effects of MCE in AI on metabolic parameters (43). Using a case-control analysis, 41 patients with AI were compared to controls matched for sex, age and BMI. Using a 75-g OGTT as opposed to the gold standard hyperinsulinemic-euglycemic clamp, higher insulin resistance and a higher glucose excursion at up to 2 hours post OGTT was seen in patients with AI and a post 1 mg ONDST serum cortisol >138 nmol/L in this small (n=12) cohort. It is difficult to be certain from these small observational studies, however, if MCE in AI is mechanistically driving insulin resistance or alternatively, the known mitogenic and proliferative effects of insulin (42) result in a higher prevalence of AI and subsequent MCE in those with pre-established hyperinsulinemia. In addressing causation, a methodologically robust study has investigated whether the hormonal and morphological features of AI correlate with insulin resistance using the hyperinsulinemic-euglycemic clamp (44). In a cohort of 42 patients with AI, 7 had MCE defined as post 1 mg ONDST serum cortisol >83 nmol/L whilst the majority had NFAI. Interestingly, a direct correlation was observed between insulin resistance and adrenal lesion size in NFAI. Given the relatively high cut-off used for defining MCE it is possible that some in the NFAI group had modest elevations of post dexamethasone suppression serum cortisol (>50 but <83 nmol/L) and that at these levels of mild hypercortisolism, chronic hyperinsulinemia is present and may increase lesion size through mitotic effects.
The well-known effects of glucocorticoids on glucose hemostasis also make a causal relationship between MCE in AI and altered glucose metabolism plausible. Cortisol at the hepatic levels promotes gluconeogenesis, peripherally in skeletal muscles reduces insulin mediated glucose uptake by interfering with insulin signaling and directly inhibits insulin release from pancreatic β cells (45). Cumulative tissue exposure to excess cortisol albeit mild may help explain why patients with AI develop compensatory hyperinsulinemia, impaired glucose tolerance and frank T2D (46).
Insulin resistance is also universally found in non-alcoholic fatty liver disease (NAFLD): a leading cause of chronic liver disease requiring transplantation (47,48). A prospective study investigated NAFLD using abdominal CT and homoeostasis model assessment for insulin resistance post a 2-hour OGTT in 56 participants with AI and 30 age, sex and BMI matched controls (49). Whilst there was no significant difference in CT attenuation scores for NAFLD in those with AI compared to controls, indices of insulin resistance were higher in the AI group. A small subgroup of patients with AI (12 of 56) demonstrated evidence of MCE. In this group, there was a trend towards a higher prevalence of NAFLD compared to eucortisolaemic controls but it is likely that the study was underpowered to test this difference robustly (49). Adequately powered studies using more sensitive imaging modalities to detect NAFLD such as magnetic resonance spectroscopy (50) are needed.
Visceral fat
MCE in AI may also impair glucose metabolism through mechanisms independent of carbohydrate metabolism. Visceral obesity is a strong risk factor for T2D and accumulation of visceral fat significantly increases insulin resistance (51). Glucocorticoid receptors are more richly expressed in visceral adipose tissue compared to subcutaneous fat (52-54). Cortisol stimulates lipoprotein lipase activity resulting in adipocyte differentiation and triglyceride synthesis which can drive insulin resistance (55). In a comprehensive retrospective cohort study, Debono et al. systematically measured visceral fat using CT in 125 patients with AI and correlated fat distribution with post 1 mg ONDST serum cortisol values (12). Visceral fat was significantly increased in 68 patients with post ONDST cortisol value >50 nmol/L similar to that seen in overt CS compared to those with a post suppression cortisol <50 nmol/L (P=0.04). This study was limited as no additional data on glucose metabolism was collected, there were no non-AI controls and it was retrospective in design. Nonetheless, these data suggest a threshold effect for a visceral fat phenotype in AI with MCE which is an important, potentially reversible, metabolic risk factor.
Another more recent retrospective cohort study from the United States has evaluated body fat composition using CT and compared three groups: overt CS (n=25), MCE with AI (n=48) and NFAI (n=32) (56). Morning cortisol values post 1 mg ONDST were significantly higher in the overt Cushing’s group compared to MCE and NFAI (441 vs. 102 vs. 39 nmol/L, respectively; P<0.001). Interestingly, in this cohort only patients with overt CS had higher visceral/total fat ratio (P<0.001) and visceral/subcutaneous fat ratio (P<0.001) when compared to patients with NFAI. The same body composition ratios were reduced in patients with MCE (P=0.007 and P=0.01 respectively) when compared to the NFAI. Post 1 mg ONDST serum cortisol values, however, correlated positively with an increase in visceral fat. It is possible that in this study the NFAI group was “contaminated” with those that had evidence of subtle cortisol excess as suggested by relatively suppressed median ACTH of 2.2 pmol/L in the NFAI cohort. Also, results could have been confounded by duration of cortisol exposure prior to diagnosis between groups and polymorphisms in glucocorticoid receptors between groups that could serve to increase or decrease receptor sensitivity and thus modulate effects on visceral adiposity.
Dyslipidemia
Dyslipidemia is a key feature of the metabolic syndrome (57). Longitudinal follow-up studies (11,58) and a recent meta-analysis (10) of 32 studies with at least 1 year of follow-up has shown an increased prevalence of dyslipidemia in those with AI and MCE. Elevated triglycerides (43) in addition to an elevated total cholesterol, LDL cholesterol and decreased high-density lipoprotein (HDL) cholesterol levels have been reported (35,59). Data suggest that excess cortisol can influence both directly and indirectly: hepatic very low-density lipoprotein synthesis, hepatic fat accumulation, lipolysis and free fatty acid production and turnover as plausible mechanisms to explain lipid abnormalities (60).
It is not clear from observational data, however, if MCE is directly driving dyslipidemia or rather whether hyperinsulinemia, impaired glucose metabolism and visceral obesity alters lipid metabolism. A study of 338 patients with AI examined the influence of MCE on lipid metabolism in the presence or absence of impaired glucose metabolism (61). The authors report that in the absence of impaired glucose metabolism, excess cortisol per se did not alter lipid metabolism. These data imply that MCE in AI results in hyperinsulinemia and impaired glucose metabolism which in turn promotes dyslipidemia.
Management of MCE
Surgical
Several studies have reported the cardiovascular benefits of adrenalectomy in AI (21,22,24) although others have shown no significant effect (20). The majority of data available comes from heterogeneous observational studies with only one randomized controlled trial available.
One group reported a significant improvement in blood pressure and fasting glucose in MCE patients treated surgically compared to those conservatively managed who more frequently experienced worsening of blood pressure, glucose and low-density lipoproteins (21). This study is limited by a small sample size and a relatively high serum cortisol cut-off of 83 nmol/L post ONDST used to define MCE which increases specificity but also increases the risk of false negatives. In addition, patient age was significantly lower in the treated group (P<0.01) suggesting a selection bias (21).
There is only one surgical study that was prospective and randomized but recruited over 15 years (22). Toniato et al. studies 45 patients and randomly assigned 23 patients with MCE to undergo laparoscopic adrenalectomies and 22 patients to conservative medical management with a mean follow-up of 7.7 years (22). MCE was defined as a serum cortisol of >69 nmol/L post 1 mg ONDST. There was a significant improvement in hypertension only in the surgical group with a normalization or improvement noted in 67% (P=0.046). Deterioration in hypertension, diabetes and dyslipidemia was noted in the conservative group although no statistical significance was observed as the study was underpowered to detect between group differences (22).
In a larger sample size of 70 patients with MCE defined as serum cortisol >50 nmol/L post 1 mg ONDST, 26 patients treated surgically and 44 conservatively were compared (23). At baseline the age of the treatment group was significantly lower than the conservative group suggesting selection bias. The authors report a significant reduction in arterial hypertension, diabetes, obesity and metabolic syndrome in those undergoing a unilateral laparoscopic adrenalectomy (P<0.05) (23).
An improvement in blood pressure post-surgery was also noted by Erbil et al. but with no significant change in diabetes, hyperlipidemia or BMI although the sample size of 11 patients with MCE may not be representative of the true population (20).
A recent meta-analysis concluded that based on the low to moderate quality evidence available, there was an improvement in cardiovascular morbidities in a significant percentage of patients post adrenalectomy (24). The ESE guidelines recommend a unilateral adrenalectomy in patients with a post 1 ONDST serum cortisol >138 nmol/L and comorbidities associated with cortisol excess (4). It is important to note, however, that cardiometabolic co-morbidities associated with MCE have a high prevalence in the general population and the incidence increases with age. It can thus be difficult in clinical practice to dissect if mild biochemical hypercortisolism from an autonomously functioning AI is directly driving co-morbidities. Selection for surgery is therefore an art and requires careful consideration of these factors and discussion in a multidisciplinary team. If contemplated, it is mandatory surgery is performed by an experienced surgeon with a sufficient volume of adrenal surgery and laparoscopic expertise (62).
Medical
We have conducted two proof-of-principle studies of medical therapies in MCE. In a prospective open label study, mifepristone reduced insulin resistance in patients with AI as measured by decreased insulin area under curve (AUC) (63). A further detailed, prospective, proof-of-concept study found patients with AI to have an abnormal circadian rhythm with excess nocturnal cortisol exposure and increased levels of IL-6 which have been shown to be associated with low grade inflammation and CVE (64). Metyrapone administered in the evening led to a reduction in AUC cortisol and IL-6 levels resetting the abnormal circadian rhythm to “normal” (65). Both studies were limited by a small sample size, use of surrogate end-points and lack of follow-up. Further research is needed to determine if pharmacotherapy to lower or off-set the effect of MCE in AI can prove beneficial on cardiometabolic end-points.
Conclusions
Data derived from observational studies indicates that MCE in the context of AI is associated with a wide array of cardiovascular and metabolic effects and an increase in mortality. A number of biologically plausible mechanisms have been proposed to explain these findings. It is important to note, however, that these mechanistic insights are largely obtained from the study of overt CS where tissue cortisol exposure is far higher. At present, it is difficult to establish causality between MCE and adverse cardiovascular and metabolic effects for several reasons. First, most studies have studied older patients and both cardiovascular and metabolic co-morbidities and AI increase in incidence with age. Second, studies examining the mal effects of MCE have been mainly retrospective in design which makes it challenging to dissect causation from association. Third, where prospective data is available, it is limited by heterogeneity in the selection criteria of participants especially in defining MCE based on serum cortisol cut-offs and this precludes a meaningful comparison between studies.
It also remains clinically challenging to manage MCE in AI due to a lack of robustly designed randomized controlled trials that test the effects of conservative versus surgical treatment on cardiometabolic complications. These data are urgently needed. Future research also needs to focus on risk stratification of those with MCE based not only on age, existing co-morbidities and cortisol excess but also genetic polymorphisms in the glucocorticoid receptor gene that determine the effects of cortisol on target tissues. A personalized approach combining genetic and clinical data will allow judicious patient selection for surgery whilst those at low risk of complications can be managed conservatively. Currently, recognition of the effects of MCE in AI on both cardiovascular and metabolic systems is important so that these complications can be screened for and diagnosed. Management of patients should be discussed in a multidisciplinary team and where surgery is considered it must be performed by experienced surgeons within a high-volume practice. In those treated conservatively, regular re-evaluation for a deterioration in cardiometabolic parameters is reasonable and surgery can be re-considered with clinical and biochemical progression.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: J Newell-Price has research and consultancy (paid to the University of Sheffield) from HRA Pharma and Novartis. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young WF. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 2007;356:601-10. [Crossref] [PubMed]
- Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass ("incidentaloma"). Ann Intern Med 2003;138:424-9. [Crossref] [PubMed]
- Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest 2006;29:298-302. [Crossref] [PubMed]
- Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2016;175:G1-34. [Crossref] [PubMed]
- Yener S. Metabolic and cardiovascular impact of non-functioning adrenal adenomas: a clinical dilemma. Eur J Intern Med 2013;24:520-4. [Crossref] [PubMed]
- Terzolo M, Pia A, Reimondo G. Subclinical Cushing's syndrome: definition and management. Clin Endocrinol (Oxf) 2012;76:12-8. [Crossref] [PubMed]
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008;93:1526-40. [Crossref] [PubMed]
- Chiodini I. Clinical review: Diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab 2011;96:1223-36. [Crossref] [PubMed]
- Di Dalmazi G, Pasquali R, Beuschlein F, et al. Subclinical hypercortisolism: a state, a syndrome, or a disease? Eur J Endocrinol 2015;173:M61-71. [Crossref] [PubMed]
- Elhassan YS, Alahdab F, Prete A, et al. Natural History of Adrenal Incidentalomas With and Without Mild Autonomous Cortisol Excess: A Systematic Review and Meta-analysis. Ann Intern Med 2019;171:107-16. [Crossref] [PubMed]
- Morelli V, Reimondo G, Giordano R, et al. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab 2014;99:827-34. [Crossref] [PubMed]
- Debono M, Prema A, Hughes TJ, et al. Visceral fat accumulation and postdexamethasone serum cortisol levels in patients with adrenal incidentaloma. J Clin Endocrinol Metab 2013;98:2383-91. [Crossref] [PubMed]
- Debono M, Bradburn M, Bull M, et al. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab 2014;99:4462-70. [Crossref] [PubMed]
- Di Dalmazi G, Vicennati V, Garelli S, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing's syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol 2014;2:396-405. [Crossref] [PubMed]
- Di Dalmazi G, Vicennati V, Rinaldi E, et al. Progressively increased patterns of subclinical cortisol hypersecretion in adrenal incidentalomas differently predict major metabolic and cardiovascular outcomes: a large cross-sectional study. Eur J Endocrinol 2012;166:669-77. [Crossref] [PubMed]
- Morelli V, Arosio M, Chiodini I. Cardiovascular mortality in patients with subclinical Cushing. Ann Endocrinol (Paris) 2018;79:149-52. [Crossref] [PubMed]
- Iacobellis G, Petramala L, Barbaro G, et al. Epicardial fat thickness and left ventricular mass in subjects with adrenal incidentaloma. Endocrine 2013;44:532-6. [Crossref] [PubMed]
- Becker J, Woloszyn J, Bold R, et al. The Adrenal Incidentaloma: An Opportunity to Improve Patient Care. J Gen Intern Med 2018;33:256-7. [Crossref] [PubMed]
- Taya M, Paroder V, Bellin E, et al. The relationship between adrenal incidentalomas and mortality risk. Eur Radiol 2019;29:6245-55. [Crossref] [PubMed]
- Erbil Y, Ademoğlu E, Ozbey N, et al. Evaluation of the cardiovascular risk in patients with subclinical Cushing syndrome before and after surgery. World J Surg 2006;30:1665-71. [Crossref] [PubMed]
- Chiodini I, Morelli V, Salcuni AS, et al. Beneficial metabolic effects of prompt surgical treatment in patients with an adrenal incidentaloma causing biochemical hypercortisolism. J Clin Endocrinol Metab 2010;95:2736-45. [Crossref] [PubMed]
- Toniato A, Merante-Boschin I, Opocher G, et al. Surgical versus conservative management for subclinical Cushing syndrome in adrenal incidentalomas: a prospective randomized study. Ann Surg 2009;249:388-91. [Crossref] [PubMed]
- Petramala L, Cavallaro G, Galassi M, et al. Clinical Benefits of Unilateral Adrenalectomy in Patients with Subclinical Hypercortisolism Due to Adrenal Incidentaloma: Results from a Single Center. High Blood Press Cardiovasc Prev 2017;24:69-75. [Crossref] [PubMed]
- Bancos I, Alahdab F, Crowley RK, et al. Therapy of endocrine disease: Improvement of cardiovascular risk factors after adrenalectomy in patients with adrenal tumors and subclinical Cushing's syndrome: a systematic review and meta-analysis. Eur J Endocrinol 2016;175:R283-95. [Crossref] [PubMed]
- Newell-Price J, Bertagna X, Grossman AB, et al. Cushing's syndrome. Lancet 2006;367:1605-17. [Crossref] [PubMed]
- Fardet L, Petersen I, Nazareth I. Risk of cardiovascular events in people prescribed glucocorticoids with iatrogenic Cushing's syndrome: cohort study. BMJ 2012;345:e4928. [Crossref] [PubMed]
- Fallo F, Budano S, Sonino N, et al. Left ventricular structural characteristics in Cushing's syndrome. J Hum Hypertens 1994;8:509-13. [PubMed]
- Sugihara N, Shimizu M, Kita Y, et al. Cardiac characteristics and postoperative courses in Cushing's syndrome. Am J Cardiol 1992;69:1475-80. [Crossref] [PubMed]
- Kamenický P, Redheuil A, Roux C, et al. Cardiac structure and function in Cushing's syndrome: a cardiac magnetic resonance imaging study. J Clin Endocrinol Metab 2014;99:E2144-53. [Crossref] [PubMed]
- Sbardella E, Minnetti M, D'Aluisio D, et al. Cardiovascular features of possible autonomous cortisol secretion in patients with adrenal incidentalomas. Eur J Endocrinol 2018;178:501-11. [Crossref] [PubMed]
- Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 2006;113:657-63. [Crossref] [PubMed]
- Halley CM, Houghtaling PL, Khalil MK, et al. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med 2011;171:1082-7. [Crossref] [PubMed]
- Evran M, Akkuş G, Berk Bozdoğan İ, et al. Carotid Intima-Media Thickness as the Cardiometabolic Risk Indicator in Patients with Nonfunctional Adrenal Mass and Metabolic Syndrome Screening. Med Sci Monit 2016;22:991-7. [Crossref] [PubMed]
- Yener S, Genc S, Akinci B, et al. Carotid intima media thickness is increased and associated with morning cortisol in subjects with non-functioning adrenal incidentaloma. Endocrine 2009;35:365-70. [Crossref] [PubMed]
- Tauchmanovà L, Rossi R, Biondi B, et al. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab 2002;87:4872-8. [Crossref] [PubMed]
- Morelli V, Palmieri S, Lania A, et al. Cardiovascular events in patients with mild autonomous cortisol secretion: analysis with artificial neural networks. Eur J Endocrinol 2017;177:73-83. [Crossref] [PubMed]
- Chiodini I, Gaudio A, Eller-Vainicher C, et al. Cortisol Secretion, Sensitivity, and Activity Are Associated With Hypertension in Postmenopausal Eucortisolemic Women. J Clin Endocrinol Metab 2019;104:4441-8. [Crossref] [PubMed]
- Chiodini I, Adda G, Scillitani A, et al. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care 2007;30:83-8. [Crossref] [PubMed]
- Patrova J, Kjellman M, Wahrenberg H, et al. Increased mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: a 13-year retrospective study from one center. Endocrine 2017;58:267-75. [Crossref] [PubMed]
- Androulakis II, Kaltsas G, Piaditis G, et al. The clinical significance of adrenal incidentalomas. Eur J Clin Invest 2011;41:552-60. [Crossref] [PubMed]
- Lopez D, Luque-Fernandez MA, Steele A, et al. "Nonfunctional" Adrenal Tumors and the Risk for Incident Diabetes and Cardiovascular Outcomes: A Cohort Study. Ann Intern Med 2016;165:533-42. [Crossref] [PubMed]
- Reincke M, Fassnacht M, Vath S, et al. Adrenal incidentalomas: a manifestation of the metabolic syndrome? Endocr Res 1996;22:757-61. [Crossref] [PubMed]
- Terzolo M, Pia A, Ali A, et al. Adrenal incidentaloma: a new cause of the metabolic syndrome? J Clin Endocrinol Metab 2002;87:998-1003. [Crossref] [PubMed]
- Muscogiuri G, Sorice GP, Prioletta A, et al. The size of adrenal incidentalomas correlates with insulin resistance. Is there a cause-effect relationship? Clin Endocrinol (Oxf) 2011;74:300-5. [Crossref] [PubMed]
- Giordano R, Guaraldi F, Berardelli R, et al. Glucose metabolism in patients with subclinical Cushing’s syndrome. Endocrine 2012;41:415-23. [Crossref] [PubMed]
- Scaroni C, Zilio M, Foti M, et al. Glucose Metabolism Abnormalities in Cushing Syndrome: From Molecular Basis to Clinical Management. Endocr Rev 2017;38:189-219. [Crossref] [PubMed]
- Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2006;91:4753-61. [Crossref] [PubMed]
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006;43:S99-112. [Crossref] [PubMed]
- Papanastasiou L, Pappa T, Samara C, et al. Nonalcoholic fatty liver disease in subjects with adrenal incidentaloma. Eur J Clin Invest 2012;42:1165-72. [Crossref] [PubMed]
- Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005;288:E462-8. [Crossref] [PubMed]
- Gabriely I, Ma XH, Yang XM, et al. Removal of Visceral Fat Prevents Insulin Resistance and Glucose Intolerance of Aging. An Adipokine-Mediated Process? Diabetes 2002;51:2951-8. [Crossref] [PubMed]
- Rebuffé-Scrive M, Lundholm K, Bjorntorp P. Glucocorticoid hormone binding to human adipose tissue. Eur J Clin Invest 1985;15:267-71. [Crossref] [PubMed]
- Fox CS, Massaro JM, Hoffmann U, et al. Abdominal Visceral and Subcutaneous Adipose Tissue Compartments. Circulation 2007;116:39-48. [Crossref] [PubMed]
- Vega GL, Adams-Huet B, Peshock R, et al. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab 2006;91:4459-66. [Crossref] [PubMed]
- Seckl JR, Morton NM, Chapman KE, et al. Glucocorticoids and 11beta-hydroxysteroid dehydrogenase in adipose tissue. Recent Prog Horm Res 2004;59:359-93. [Crossref] [PubMed]
- Delivanis DA, Iniguez-Ariza NM, Zeb MH, et al. Impact of hypercortisolism on skeletal muscle mass and adipose tissue mass in patients with adrenal adenomas. Clin Endocrinol (Oxf) 2018;88:209-16. [Crossref] [PubMed]
- Kolovou GD, Anagnostopoulou K, Cokkinos D. Pathophysiology of dyslipidaemia in the metabolic syndrome. Postgrad Med J 2005;81:358-66. [Crossref] [PubMed]
- Sereg M, Szappanos A, Toke J, et al. Atherosclerotic risk factors and complications in patients with non-functioning adrenal adenomas treated with or without adrenalectomy: a long-term follow-up study. Eur J Endocrinol 2009;160:647-55. [Crossref] [PubMed]
- Rossi R, Tauchmanova L, Luciano A, et al. Subclinical Cushing's syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab 2000;85:1440-8. [PubMed]
- Arnaldi G, Scandali VM, Trementino L, et al. Pathophysiology of dyslipidemia in Cushing's syndrome. Neuroendocrinology 2010;92 Suppl 1:86-90. [Crossref] [PubMed]
- Masserini B, Morelli V, Palmieri S, et al. Lipid abnormalities in patients with adrenal incidentalomas: role of subclinical hypercortisolism and impaired glucose metabolism. J Endocrinol Invest 2015;38:623-8. [Crossref] [PubMed]
- Park HS, Roman SA, Sosa JA. Outcomes from 3144 adrenalectomies in the United States: which matters more, surgeon volume or specialty? Arch Surg 2009;144:1060-7. [Crossref] [PubMed]
- Debono M, Chadarevian R, Eastell R, et al. Mifepristone reduces insulin resistance in patient volunteers with adrenal incidentalomas that secrete low levels of cortisol: a pilot study. PLoS One 2013;8:e60984. [Crossref] [PubMed]
- Ridker PM, Hennekens CH, Buring JE, et al. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N Engl J Med 2000;342:836-43. [Crossref] [PubMed]
- Debono M, Harrison RF, Chadarevian R, et al. Resetting the Abnormal Circadian Cortisol Rhythm in Adrenal Incidentaloma Patients With Mild Autonomous Cortisol Secretion. J Clin Endocrinol Metab 2017;102:3461-9. [Crossref] [PubMed]


