
Surgical approach to patients with hypercortisolism
ACTH-dependent hypercortisolism
In ACTH-dependent hypercortisolism, treatment focuses on the surgical removal of the primary source of ACTH production. However, when the source is occult, surgical removal is not feasible or resection and/or debulking fails to control the disease, bilateral adrenalectomy can be considered.
Pituitary ACTH-dependent hypercortisolism
Hypercortisolism caused by an ACTH-secreting pituitary adenoma (Cushing’s disease, CD) is the most common cause (70%) of endogenous hypercortisolism (1). The goals of treatment are to achieve remission of disease and long-term control without recurrence. Transsphenoidal microsurgery is the recommended first line treatment for pituitary overproduction of ACTH (2). Transsphenoidal surgery (TSS) achieves remission in 42–97% of cases (1). In cases in which surgery fails, repeat TSS, radiotherapy, medical therapy, and/or bilateral adrenalectomy are options for management. Extensive discussion of pituitary reoperation and/or medical therapy of CD are beyond the scope of this chapter.
Bilateral adrenalectomy for CD has nearly a 100% success rate and results in immediate control of steroid excess. However, it results in lifelong dependency on glucocorticoid substitution and the risk of developing Nelson’s syndrome and therefore should only be considered when all other management options have been exhausted for persistent or recurrent CD. Nelson’s syndrome is a rare clinical manifestation that occurs in 8–47% of patients after bilateral adrenalectomy. It is associated with the clinical trial of hyperpigmentation, excessive ACTH secretion, and a pituitary adenoma (3). Table 1 describes the management options available in patients with CD after failure of TSS as well as the perceived advantages and disadvantages of each option.
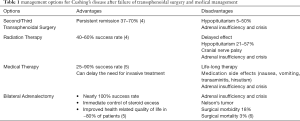
Full table
Ectopic ACTH-dependent hypercortisolism
One of the more challenging causes of hypercortisolism for the adrenal surgeon is ectopic ACTH-dependent hypercortisolism or ectopic ACTH Cushing’s syndrome (EAS). EAS comprises 5% to 20% of all cases of ACTH-dependent hypercortisolism (7-9). EAS is commonly associated with neuroendocrine tumors (NETs) such as bronchial, pancreatic and thymic carcinoids, as well as, small cell lung carcinomas, medullary thyroid carcinoma, and pheochromocytomas. However, occult tumors occur in approximately 12–38% of the reported series making EAS difficult to treat because the primary tumor cannot be localized (7).
The primary treatment goal in patients with EAS is to remove the source of autonomous ACTH without permanent disruption of the pituitary-adrenal axis (4). When the ectopic source of ACTH is identified the optimal treatment is extirpation of the primary or metastatic tumor. In patients with a single primary tumor, resection has been associated with an 83% remission rate (7,10,11). Overall, attempts at removal of the ACTH tumor source fails in over 50% of patients, partly due to unresectable metastases and also because many tumors are small and therefore difficult to localize (12). Thus, a substantial number of patients (30–56%) ultimately undergo bilateral adrenalectomy when the EAS source remains occult and/or the hypercortisolism cannot be treated by other means (7,10,11).
Prior to proceeding with bilateral adrenalectomy for the management of EAS, every effort should be made to localize the primary or metastatic source. As these tumors can occur in multiple sites, thin-cut cross sectional imaging (CT or MRI) of the chest, abdomen and pelvis is recommended as the initial imaging modality (7). However the sensitivity for detection for the source of EAS is low, ranging from 52–66% (11,13). Whereas previous studies utilizing [(18)F]fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging have concluded that FDG-PET was not useful in localizing tumors that were occult on CT or MRI (14), others have concluded that FDG-PET was helpful in localizing the source of ACTH in small-cell lung NETs, atypical thymic tumors and medullary thyroid carcinoma but not helpful in well differentiated or low grade NETs (15).
Recently, in a multi-institutional retrospective series of 28 patients with EAS the use of PET/CT with [68Ga]‐DOTATATE (DOTA‐(Tyr3)‐octreotate) was shown to localize the primary tumor and/or metastases or change management in the majority of study patients (18/28, 64%). In this study, 11/17 (65%) of previously occult tumors were identified using [68Ga]‐DOTATATE (12). Thus, [68Ga]‐DOTATATE may prove to be a useful adjunct in localizing and managing previously occult ACTH producing tumors. Consideration of additional organ specific imaging such as thyroid ultrasound or endoscopic ultrasound of the pancreas can be entertained based upon clinical suspicion, the results of other imaging studies (CT, MRI, [68Ga]‐DOTATATE or FDG PET/CT) results or biochemical evaluation. To our knowledge the current guidelines (ENDO and NCCN) have not clearly recommended and imaging algorithm for AES patients.
Once all localizing modalities have been exhausted and/or medical management or surgical resection/debulking of the primary or metastatic tumor has failed, surgical bilateral adrenalectomy may be reasonable in select patients. Ideally, this is performed in an elective setting, however occasionally in severe EAS the need for adrenalectomy becomes more urgent. This is typically encountered when a patient’s cortisol elevation results in significant end organ effects such as sepsis, severe and intractable hypokalemia, uncontrolled hypertension, heart failure, gastrointestinal hemorrhage, glucocorticoid-induced acute psychosis, progressive debilitating myopathy, thromboembolism, and/or uncontrolled hyperglycemia (4). It is essential to stabilize these patients and manage the end organ effects prior to proceeding to the operating room. Medical management of hypercortisolism may include a combination of mitotane, metyrapone, ketoconazole and/or etomidate to inhibit steroidogenesis.
Bilateral adrenalectomy for ACTH-dependent hypercortisolism operative considerations
Bilateral adrenalectomy is currently considered a second or third line treatment for ACTH-dependent hypercortisolism, either CD or EAS (16). Since the introduction laparoscopic adrenalectomy in 1992, minimally invasive surgery (MIS) of the adrenal gland has become the preferred approach for bilateral adrenalectomy. Minimally invasive approaches include laparoscopic transabdominal (LTA), posterior retroperitoneoscopic (PRA), and robotic-assisted TA and PRA (RTA or RPRA). MIS has been associated with less blood loss and shorter hospital stays when compared to open approaches (17). While no individual MIS approach has been demonstrated to be superior, there are certainly advantages and disadvantages to each (Table 2). A review of 23 studies that included 739 patients who underwent bilateral adrenalectomy (approximately 70% performed laparoscopically) reported a surgical morbidity of 18% and 30-day mortality of 3% (6).
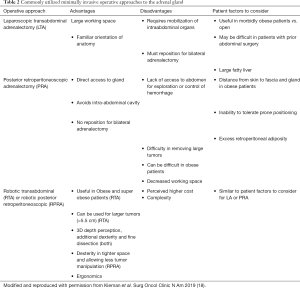
Full table
While some centers advocate for bilateral PRA to avoid patient repositioning, in our experience many of these patients are better candidates for bilateral TA adrenalectomy which can be performed laparoscopic or robotic safely and efficiently. The PRA approach can be difficult in this patient population for two reasons: (I) central obesity, abdominal wall thickness and the marked increase in retroperitoneal fat making access and visualization difficult and (II) the majority of these patients are medically unfit and may not tolerate the prone position for a prolonged period. The authors also feel that the TA approach may allow for a more complete wide adrenalectomy including a generous amount of en-bloc periadrenal fat given the more familiar anatomy and less constricted space. Failure to remove all adrenal tissue in these patients leads to persistent hypercortisolism. PRA remains a feasible approach in a select group of these patients.
Bilateral adrenalectomy for ACTH-dependent hypercortisolism postoperative considerations
The resection of both adrenal glands causes permanent adrenal insufficiency resulting in the lifelong need for glucocorticoid and mineralocorticoid replacement. Patients are typically given “stress-dose” steroids in the perioperative period. In our institution we use hydrocortisone 50 mg intravenously every 8 hours. Once the patient is tolerating oral intake they are transitioned to oral hydrocortisone. Most patients with adrenal insufficiency require 15–25 mg of hydrocortisone daily in split doses. The first dose should be taken when the patient first wakes up and the last dose in the evening (19). Most patients should take 50–200 ug of fludrocortisone as a single daily dose. If essential hypertension develops, the dose of fludrocortisone can be reduced, but not stopped (19). Surgery, invasive medical procedures or physiologic stress require administration of additional stress dose steroids.
The risk of adrenal crisis must be discussed with patients and ample education must be provided regarding signs and symptoms of adrenal insufficiency and adrenal crisis and the necessity of glucocorticoid and mineralocorticoid compliance and dose adjustment in times of stress. Medical alert jewelry should be required to alert medical personnel in case of loss of consciousness occurs in a time of adrenal insufficiency. Additionally, patients should have additional doses of hydrocortisone tablets as well as an injectable hydrocortisone formulation in case of emergency. Patients should be instructed to double the daily hydrocortisone dose in times of illness/stress and notify health care providers if they are unable to tolerate PO medications due to gastrointestinal disturbances.
Adrenal crisis is a life-threatening emergency that requires immediate recognition and treatment. Approximately 8% of patients with adrenal insufficiency will experience an adrenal crisis each year (20-22). This most commonly occurs during a gastrointestinal illness but can also be precipitated by infection, invasive procedures, injuries, physical activity, pregnancy and psychological stress (23). Prompt medical attention with immediate intramuscular or intravenous high dose hydrocortisone (100 mg) and intravenous fluids is essential.
It is also important for clinicians caring for patients after bilateral adrenalectomy to note that the preoperative hypercortisolism can impact the patient’s immunity and increase the risk of postoperative infectious complications. Additionally hypercortisolism alters the coagulation cascade for up to 1 year after surgical cure (16,24) and is associated with increased risk of deep vein thrombosis (DVT), especially in the first postoperative month (24-26). Thus prophylactic anticoagulation should be considered.
Monitoring for persistent or recurrent disease is an important aspect of postoperative management of EAS and CD patients undergoing bilateral adrenalectomy. In a review of 23 studies that included 739 patients who underwent bilateral adrenalectomy 3–34% patients had residual cortisol secretion due to accessory adrenal tissue or adrenal remnants, but less than 2% had a relapse of hypercortisolism (6). Rarely, residual adrenal cortical tissue regrows in the surgical bed or even more rarely in the gonads as a result of prolonged ACTH hypersecretion. If a patient develops signs or symptoms of hypercortisolism after bilateral adrenalectomy, clinicians should test endogenous cortisol secretion by withholding steroid replacement for 24 hours and checking morning serum cortisol levels (16).
ACTH-independent hypercortisolism
The majority ACTH-independent or adrenal based cases of hypercortisolism are due to unilateral adrenal adenomas. Much less frequently occurring etiologies of ACTH-independent hypercortisolism are bilateral adrenal macronodular hyperplasia (BAMH), primary pigmented adrenal disease (PPAD) and adrenal cortical carcinoma (ACC). In this section we will discuss the preoperative, operative and postoperative considerations for adrenal surgeons treating ACTH-independent hypercortisolism.
Unilateral adrenal adenoma
Unilateral adrenal adenomas are benign neoplasms of the adrenal gland that account for approximately 60% of adrenal causes of hypercortisolism (27). They are often incidentally discovered on cross sectional imaging but may also be localized after a patient presents with signs and symptoms or Cushing’s syndrome and biochemical evaluation is consistent with an adrenal source of hypercortisolism.
After the diagnosis has been confirmed and the gland has been localized with cross-sectional imaging, the recommended management for adrenal hypercortisolism is unilateral adrenalectomy (see below for exceptions and rare conditions).
As previously discussed, most patients with adrenal tumors can undergo a MIS. Such approach is associated with shorter hospitalization, less pain and morbidity, quicker recovery, and low incidence of incisional hernias. An absolute contraindication to MIS adrenalectomy is extension of the tumor into nearby structures. A relative contraindication is tumor size when ACC is highly suspected. From a practical standpoint, tumors >6 to 8 cm may be more difficult to mobilize and manipulate and can often result in conversion to an open procedure. Additionally, as discussed in the subsequent section adrenal tumors >4–6 cm that produce cortisol and are indeterminate on cross sectional imaging warrant careful consideration of the operative approach due to the increased risk of ACC.
BAMH
BAMH is very rare cause of ACTH independent hypercortisolism. It presents on imaging with characteristic multiple bilateral macronodules (>10 mm) with hyperplasia and/or internodular atrophy. It is often diagnosed as bilateral adrenal incidentalomas with autonomous cortisol secretion (ACS) (28,29).
The treatment of choice to control hypercortisolism in patients with BAMH is bilateral adrenalectomy. However, studies have demonstrated some evidence of biochemical and clinical improvement with unilateral adrenalectomy. A recent study by Osswald et al. compared 25 patients with BAMH who underwent unilateral adrenalectomy to 9 patients who underwent bilateral adrenalectomy and found that 84% of patients who underwent unilateral adrenalectomy had initial biochemical remission but only 32% of patients remained in biochemical remission at last follow-up with median follow-up of 50 months, compared to 100% in the bilateral adrenalectomy group. The rates of adrenal insufficiency were much lower in the unilateral adrenalectomy group (50% temporary, 5% permanent) compared to bilateral adrenalectomy (100%) for BAMH. Thus the authors conclude that in select patients with asymmetric hyperplasia or mild cortisol secretion it is reasonable to consider unilateral adrenalectomy as acceptance of mild hypercortisolism may be preferable to avoid lifelong glucocorticoid dependence and risk of adrenal crisis (30). The authors have performed unilateral adrenalectomy in BAMH with good results, always educating patients that a contralateral adrenalectomy may be necessary. How to choose which adrenal to remove first is purely based on the size of the adrenal gland and nodules, removing the larger gland first. In order to discern which adrenal gland is contributing the largest amount of cortisol, adrenal venous sampling has been performed (31), but to date results are disappointing. These complicated patients should be cared for in a multidisciplinary fashion in close collaboration with a pituitary/adrenal expert endocrinologist.
PPAD
PPAD is a rare cause of ACTH independent hypercortisolism occurring mainly in children and young adults. Its name originates from the macroscopic appearance of the adrenal gland as it has characteristic small pigmented molecules in the cortex. The disease is associated with the Carney complex which is a complex of cardiac myxoma, pituitary adenoma, thyroid adenoma or carcinoma, testicular tumors, ovarian cysts, schwannomas and breast adenomas (32). On imaging the adrenals can appear normal or micronodules can be visible on one or both glands. The definitive treatment of choice to control hypercortisolism in patients with PPAD is bilateral MIS adrenalectomy (16).
ACC
ACC is a rare and aggressive primary adrenal malignancy (33,34). It is commonly diagnosed during evaluation of symptoms related to hormone excess as approximately 60% of ACC’s release hormones, most frequently cortisol (20–30%) (35-37). Manifestations of hypercortisolism in ACC can be subclinical or overt and/or paired with androgen excess. Cortisol excess in ACC has been associated with decreased overall and recurrence free survival in retrospective studies (38). And as discussed above, it has also been associated with increased risk of postoperative complications due to the impact on the immune system, coagulation cascade and tissue healing (36).
The possibility of ACC should be considered in all patients with an indeterminate adrenal mass and hypercortisolism. While some patients will present with symptoms of malignancy such as weight loss, anorexia, cachexia or pain, a large proportion will not present with symptoms and therefore a review of preoperative imaging is essential to evaluate the risk of malignancy.
Imaging of an adrenal mass suspicious for ACC should include an adrenal protocol CT or MRI. On CT, ACC is usually large and heterogeneous with indistinct borders. Locally invasion of adjacent organs is common. Invasion of liver, kidney and vena cava is common on the right, whereas pancreas, spleen, renal vein and kidney may be involved on the left. On venous phase imaging vena cava tumor thrombus may also be identified, often originating at the level of the right adrenal vein and extending into the retrohepatic cava or extending from the left renal vein into the infrahepatic cava. If ACC is suspected, a CT of the chest should be performed to evaluate for pulmonary metastasis and identify any occult pulmonary emboli. FDG PET can also be used to help in the diagnosis and staging of patients with suspected ACC (18).
Complete surgical resection with negative margins is the only curative option for ACC (33,39-42). Thus, the operative approach chosen must have the highest likelihood of achieving this goal. The AACE/AAES, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) as well as the European Network for the Study of Adrenal Tumors (ENSAT) guidelines all agree that open adrenalectomy should be performed if ACC is suspected (43-45). These recommendations are due in part to the nature of ACC.
ACC is often a very soft and necrotic tumor; capsular disruption and fragmentation are easy to induce, particularly during direct tumor manipulation that commonly occurs in MIS. Furthermore, ACC tends to invade through the tumor capsule with microscopic disease present at the gland surface, thus minimizing direct contact with the tumor surface is essential to avoid violating the tumor capsule or causing disruption of disease at the surface of the gland (33). Thus, we agree with the aforementioned consensus guidelines and perform open adrenalectomy for known or suspected ACC.
Finally, the importance of surgeon experience in management of ACC cannot be overemphasized. Several studies have demonstrated higher rates of complete resection and improved outcomes when ACCs are managed by surgeons or at centers with expertise (40,46-49). Referral to high volume centers, experienced in treating patients with adrenal tumors and ACC, should be considered prior to proceeding with resection, regardless of operative approach.
The role of robotic adrenal surgery in management of patients with hypercortisolism and benign adrenal tumors
The first robotic adrenalectomy was reported by Horgan et al. in 2001 (50) and since that time has gained popularity due to the improved optics associated with 3D vision, the freedom of movement from the Endo-wrist instrumentation and improved surgeon ergonomics (51). While some argue that robotic technology is associated with higher cost and prolonged operative times (52) others have demonstrated that by limiting unnecessary instruments and energy devices as well as having an experience surgical team the cost and operative times can be equivalent (51).
As previously mentioned, in patients with hypercortisolism, there is often increased central adiposity which can make traditional laparoscopic surgery quite challenging particularly when utilizing a retroperitoneal approach due to its inherently constrained space. The sharp 3D visibility, ability to reach and visualize tight places and the increased dexterity of the instruments helps the surgeon overcome space constraints both for retraction and dissection. It is the authors opinion that robotic adrenalectomy is easier to learn when compared to laparoscopic. For the already experienced adrenal team we did not observe a steep learning curve when our practice switched from laparoscopic to robotic systems to remove the adrenal gland 5 years ago; and in fact, operative times decreased over time specially when the Xi da Vinci system was introduced (53). We have observed trainees perform the steps of the RTA procedure with ease and efficiency when the robot is utilized (personal observation). Robot systems allow for easier handling of the rare complication of adrenal vein tear or injury to large veins as the surgeon can quickly repair the vessel with the added dexterity of the robotic system. Lastly, robotic systems allow the authors to address larger masses, those that sit on the renal vessels in the kidney hilum and/or occasional paragangliomas in difficult spaces that may not be easy to approach laparoscopically. In essence, we are able to “push the envelope” in our ability to remove the adrenal gland.
Robotic adrenalectomy operative technique
Patients are positioned in the lateral decubitus flank position on a bean bag for the transabdominal approach (TA) and the prone jackknife position on the Wilson frame for PRA.
We acknowledge that there are many ways to place trocars in robotic procedures. Our recommended trocar placement for bilateral RTA (A), unilateral RTA (B) and unilateral RPRA (C) adrenalectomy are depicted in Figure 1. The goals of trocar placement are to triangulate to the target gland, minimize collision of the robotic arms, and allow for a functional assistant trocars ports. The Xi platform may not require the same triangulation but our trocar placement suggestion works for both systems the Si and Xi.
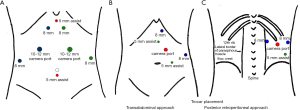
Our approach for RPRA and RTA has been described by our group previously (54) and are summarized step by step in Table 3.

Full table
Summary
The surgical management of hypercortisolism is often complex. In ACTH-dependent hypercortisolism, the first-line treatment focuses on the surgical removal of the site of ACTH production. Bilateral adrenalectomy should only be considered as a second line treatment for ACTH-dependent hypercortisolism, either CD or EAS and preferably after multidisciplinary discussion. ACTH-independent hypercortisolism is most commonly caused by a benign unilateral adrenal adenoma however consideration of much less frequently occurring etiologies of ACTH-independent hypercortisolism such as BAMH, PPAD and ACC is warranted in special populations to ensure appropriate surgical management. The majority of adrenalectomies performed for hypercortisolism can be performed utilizing a minimally invasive approach however if ACC is highly suspected open adrenalectomy should be performed. Robotic technology is a relatively new and useful technology when performing minimally invasive adrenalectomy. It can be utilized safely and cost effectively in experienced hands.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petersenn S, Beckers A, Ferone D, et al. Therapy of endocrine disease: outcomes in patients with Cushing's disease undergoing transsphenoidal surgery: systematic review assessing criteria used to define remission and recurrence. Eur J Endocrinol 2015;172:R227-39. [Crossref] [PubMed]
- Biller BM, Grossman AB, Stewart PM, et al. Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab 2008;93:2454-62. [Crossref] [PubMed]
- Patel J, Eloy JA, Liu JK. Nelson's syndrome: a review of the clinical manifestations, pathophysiology, and treatment strategies. Neurosurg Focus 2015;38:E14. [Crossref] [PubMed]
- Reincke M, Ritzel K, Osswald A, et al. A critical reappraisal of bilateral adrenalectomy for ACTH-dependent Cushing's syndrome. Eur J Endocrinol 2015;173:M23-32. [Crossref] [PubMed]
- Di Dalmazi G, Reincke M. Adrenal Surgery for Cushing's Syndrome: An Update. Endocrinol Metab Clin North Am 2018;47:385-94. [Crossref] [PubMed]
- Ritzel K, Beuschlein F, Mickisch A, et al. Clinical review: Outcome of bilateral adrenalectomy in Cushing's syndrome: a systematic review. J Clin Endocrinol Metab 2013;98:3939-48. [Crossref] [PubMed]
- Alexandraki KI, Grossman AB. The ectopic ACTH syndrome. Rev Endocr Metab Disord 2010;11:117-26. [Crossref] [PubMed]
- Sathyakumar S, Paul TV, Asha HS, et al. ECTOPIC CUSHING SYNDROME: A 10-YEAR EXPERIENCE FROM A TERTIARY CARE CENTER IN SOUTHERN INDIA. Endocr Pract 2017;23:907-14. [Crossref] [PubMed]
- Wajchenberg BL, Mendonca BB, Liberman B, et al. Ectopic adrenocorticotropic hormone syndrome. Endocr Rev 1994;15:752-87. [PubMed]
- Ilias I, Torpy DJ, Pacak K, et al. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab 2005;90:4955-62. [Crossref] [PubMed]
- Isidori AM, Kaltsas GA, Pozza C, et al. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab 2006;91:371-7. [Crossref] [PubMed]
- Wannachalee T, Turcu AF, Bancos I, et al. The Clinical Impact of [(68) Ga]-DOTATATE PET/CT for the Diagnosis and Management of Ectopic Adrenocorticotropic Hormone - Secreting Tumours. Clin Endocrinol (Oxf) 2019;91:288-94. [Crossref] [PubMed]
- Santhanam P, Taieb D, Giovanella L, et al. PET imaging in ectopic Cushing syndrome: a systematic review. Endocrine 2015;50:297-305. [Crossref] [PubMed]
- Pacak K, Ilias I, Chen CC, et al. The role of [(18)F]fluorodeoxyglucose positron emission tomography and [(111)In]-diethylenetriaminepentaacetate-D-Phe-pentetreotide scintigraphy in the localization of ectopic adrenocorticotropin-secreting tumors causing Cushing's syndrome. J Clin Endocrinol Metab 2004;89:2214-21. [Crossref] [PubMed]
- Guerin C, Taieb D, Treglia G, et al. Bilateral adrenalectomy in the 21st century: when to use it for hypercortisolism? Endocr Relat Cancer 2016;23:R131-42. [Crossref] [PubMed]
- Nieman LK, Biller BM, Findling JW, et al. Treatment of Cushing's Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015;100:2807-31. [Crossref] [PubMed]
- Morris LF, Harris RS, Milton DR, et al. Impact and timing of bilateral adrenalectomy for refractory adrenocorticotropic hormone-dependent Cushing's syndrome. Surgery 2013;154:1174-83; discussion 83-4. [Crossref] [PubMed]
- Kiernan CM, Lee JE. Minimally Invasive Surgery for Primary and Metastatic Adrenal Malignancy. Surg Oncol Clin N Am 2019;28:309-26. [Crossref] [PubMed]
- Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med 2014;275:104-15. [Crossref] [PubMed]
- Hahner S, Loeffler M, Bleicken B, et al. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol 2010;162:597-602. [Crossref] [PubMed]
- Pazderska A, Pearce SH. Adrenal insufficiency - recognition and management. Clin Med (Lond) 2017;17:258-62. [Crossref] [PubMed]
- White K, Arlt W. Adrenal crisis in treated Addison's disease: a predictable but under-managed event. Eur J Endocrinol 2010;162:115-20. [Crossref] [PubMed]
- Hahner S, Spinnler C, Fassnacht M, et al. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab 2015;100:407-16. [Crossref] [PubMed]
- van der Pas R, Leebeek FW, Hofland LJ, et al. Hypercoagulability in Cushing's syndrome: prevalence, pathogenesis and treatment. Clin Endocrinol (Oxf) 2013;78:481-8. [Crossref] [PubMed]
- Boscaro M, Sonino N, Scarda A, et al. Anticoagulant prophylaxis markedly reduces thromboembolic complications in Cushing's syndrome. J Clin Endocrinol Metab 2002;87:3662-6. [PubMed]
- Stuijver DJ, van Zaane B, Feelders RA, et al. Incidence of venous thromboembolism in patients with Cushing's syndrome: a multicenter cohort study. J Clin Endocrinol Metab 2011;96:3525-32. [Crossref] [PubMed]
- Newell-Price J, Bertagna X, Grossman AB, et al. Cushing's syndrome. Lancet 2006;367:1605-17. [Crossref] [PubMed]
- De Venanzi A, Alencar GA, Bourdeau I, et al. Primary bilateral macronodular adrenal hyperplasia. Curr Opin Endocrinol Diabetes Obes 2014;21:177-84. [Crossref] [PubMed]
- Lacroix A, Bourdeau I. Bilateral adrenal Cushing's syndrome: macronodular adrenal hyperplasia and primary pigmented nodular adrenocortical disease. Endocrinol Metab Clin North Am 2005;34:441-58. x. [Crossref] [PubMed]
- Oßwald A, Plomer E, Dimopoulou C, et al. Favorable long-term outcomes of bilateral adrenalectomy in Cushing's disease. Eur J Endocrinol 2014;171:209-15. [Crossref] [PubMed]
- Acharya R, Dhir M, Bandi R, et al. Outcomes of Adrenal Venous Sampling in Patients with Bilateral Adrenal Masses and ACTH-Independent Cushing's Syndrome. World J Surg 2019;43:527-33. [Crossref] [PubMed]
- Kamilaris CDC, Faucz FR, Voutetakis A, et al. Carney Complex. Exp Clin Endocrinol Diabetes 2019;127:156-64. [Crossref] [PubMed]
- Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev 2014;35:282-326. [Crossref] [PubMed]
- Varghese J, Habra MA. Update on adrenocortical carcinoma management and future directions. Curr Opin Endocrinol Diabetes Obes 2017;24:208-14. [Crossref] [PubMed]
- Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg 2001;25:891-7. [Crossref] [PubMed]
- Margonis GA, Kim Y, Tran TB, et al. Outcomes after resection of cortisol-secreting adrenocortical carcinoma. Am J Surg 2016;211:1106-13. [Crossref] [PubMed]
- Puglisi S, Perotti P, Cosentini D, et al. Decision-making for adrenocortical carcinoma: surgical, systemic, and endocrine management options. Expert Rev Anticancer Ther 2018;18:1125-33. [Crossref] [PubMed]
- Abiven G, Coste J, Groussin L, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab 2006;91:2650-5. [Crossref] [PubMed]
- Dackiw AP, Lee JE, Gagel RF, et al. Adrenal cortical carcinoma. World J Surg 2001;25:914-26. [Crossref] [PubMed]
- Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol 2010;17:263-70. [Crossref] [PubMed]
- Miller BS, Ammori JB, Gauger PG, et al. Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg 2010;34:1380-5. [Crossref] [PubMed]
- Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery 1992;112:963-70; discussion 70-1. [PubMed]
- Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2016;175:G1-34. [Crossref] [PubMed]
- Stefanidis D, Goldfarb M, Kercher KW, et al. SAGES guidelines for minimally invasive treatment of adrenal pathology. Surg Endosc 2013;27:3960-80. [Crossref] [PubMed]
- Zeiger MA, Thompson GB, Duh QY, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas: executive summary of recommendations. Endocr Pract 2009;15:450-3. [Crossref] [PubMed]
- Ayala-Ramirez M, Jasim S, Feng L, et al. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol 2013;169:891-9. [Crossref] [PubMed]
- Fassnacht M, Johanssen S, Fenske W, et al. Improved survival in patients with stage II adrenocortical carcinoma followed up prospectively by specialized centers. J Clin Endocrinol Metab 2010;95:4925-32. [Crossref] [PubMed]
- Hermsen IG, Kerkhofs TM, den Butter G, et al. Surgery in adrenocortical carcinoma: Importance of national cooperation and centralized surgery. Surgery 2012;152:50-6. [Crossref] [PubMed]
- Porpiglia F, Fiori C, Daffara F, et al. Retrospective evaluation of the outcome of open versus laparoscopic adrenalectomy for stage I and II adrenocortical cancer. Eur Urol 2010;57:873-8. [Crossref] [PubMed]
- Horgan S, Vanuno D. Robots in laparoscopic surgery. J Laparoendosc Adv Surg Tech A 2001;11:415-9. [Crossref] [PubMed]
- Feng Z, Feng MP, Feng DP, et al. A cost-conscious approach to robotic adrenalectomy. J Robot Surg 2018;12:607-11. [Crossref] [PubMed]
- Morino M, Beninca G, Giraudo G, et al. Robot-assisted vs laparoscopic adrenalectomy: a prospective randomized controlled trial. Surg Endosc 2004;18:1742-6. [Crossref] [PubMed]
- Feng Z, Feng MP, Feng DP, et al. Robotic-assisted adrenalectomy using da Vinci Xi vs. Si: are there differences? J Robot Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Feng Z, Feng MP, Levine JW, et al. Robotic retroperitoneoscopic adrenalectomy: useful modifications of the described posterior approach. J Robot Surg 2017;11:409-14. [Crossref] [PubMed]


