
Endocrine causes of hypertension in pregnancy
Introduction
Less than one to two percent of cases of hypertension during pregnancy are due to endocrine disorders, but if left unrecognized and untreated, some causes of endocrine-mediated hypertension have an almost 30% maternal and fetal mortality rate. Endocrine causes of hypertension include primary aldosteronism (PA) due to excess aldosterone, Cushing syndrome (CS) due to excess cortisol, and pheochromocytoma and paraganglioma leading to excess catecholamines. While these conditions are rare, the excessive morbidity and mortality underscore the importance of timely diagnosis and treatment.
While the importance of recognizing endocrine causes of hypertension is clear, there are many challenges to diagnosis and successful management. The physiologic changes that occur during pregnancy alter normal hormone dynamics, resulting in difficultly interpreting the biochemical assays usually used for diagnosis. Additionally, many of the preferred imaging modalities are contraindicated during pregnancy, limiting localization. Finally, typical management strategies used to treat endocrine causes of hypertension must be modified or delayed during pregnancy due to risk to the mother and the fetus. In this review, we will discuss the unique challenges of diagnosing hypertension due to excess aldosterone, cortisol or catecholamines during pregnancy and aim to provide practical insight into management strategies.
PA
PA is the most common cause of secondary hypertension in the non-pregnant population, accounting for approximately 10% of all hypertension (1-3). Despite this high prevalence, there are less than 50 cases of PA in pregnancy reported in the literature (4). This may be because PA has variable degrees of severity, and there are many changes that occur in the renin-angiotensin-aldosterone system (RAAS) during pregnancy that make the diagnosis of PA challenging.
Aldosterone maintains blood pressure and potassium balance through regulation by the RAAS. Activation of the RAAS begins with renin, which is produced by the kidney under conditions of perceived hypoperfusion or low tubular sodium and suppressed by hyper-perfusion or elevated sodium delivery to the macula densa. Renin catalyzes the first of two steps in the conversion of angiotensinogen to angiotensin II, and angiotensin II stimulates aldosterone secretion from the zona glomerulosa of the adrenal gland. Aldosterone, which is also stimulated by elevated potassium levels, acts in the kidney to increase sodium retention and blood volume while increasing urinary potassium excretion (5).
During normal pregnancy, there is activation of the RAAS due to increased production of renin from the uterus and placenta as well as increased angiotensinogen produced in the liver (6). Renin activity increases 4-fold by 10 weeks gestation and plateaus at 22 weeks gestation (7). This results in increased serum aldosterone which can reach 10-fold non-pregnant concentrations by the end of pregnancy; however, the effect of increased aldosterone is mitigated by progesterone, which is a competitive inhibitor of aldosterone at the mineralocorticoid receptor (MR) in the distal convoluted tubule and cortical collecting duct. Thus, the degree of aldosterone elevation seen in normal pregnancy does not usually result in hypertension or hypokalemia (8-10) (Figure 1).

PA has a variable course during pregnancy, with some patients diagnosed prior to pregnancy demonstrating clinical improvement (11,12) due to the anti-mineralocorticoid activity of progesterone. In other cases, hypertension and hyperkalemia are severe. Of the available case reports in the literature documenting PA during pregnancy, over 50% of cases resulted in delivery prior to 38 weeks (4,13-16). Other associated negative outcomes include intrauterine fetal demise in 9.4% of cases (17) and intrauterine growth retardation (4), as well as rare instances of maternal renal failure and maternal pulmonary edema. It is unclear if PA increases the risk of pre-eclampsia, as hypertension with proteinuria is common in hyperaldosteronism. However, in one study of 35 pregnancies in patients with glucocorticoid-remediable aldosteronism (GRA), a hereditary form of PA, there was no increased risk of pre-eclampsia (18).
As in the non-pregnant population, the first step in diagnosis of PA in pregnancy is measurement of an elevated aldosterone and a suppressed plasma renin activity (PRA) or renin mass. Both aldosterone and renin are elevated during pregnancy, thus non-pregnancy reference ranges cannot be used for screening (8). There are no validated reference ranges for aldosterone or renin during gestation, although some have suggested that a PRA of less than 4ng/mL/h is suggestive of PA (19). In the non-pregnant population, the next step in diagnosis is confirmatory testing (such as salt loading) and adrenal vein sampling (AVS) and/or imaging for subtype differentiation (20). Confirmatory testing is not recommended during pregnancy due to concerns of exacerbating the volume expansion that has already occurred in pregnancy. AVS is not recommended due to excessive radiation exposure. These limitations often mean that definitive diagnosis is delayed until after delivery, and likely contribute to the low rate of reported PA during pregnancy.
The literature reports a wide range of treatment strategies for PA during pregnancy, including no treatment, medical management and adrenalectomy. Regardless of treatment method, blood pressure control is the most critical aspect of management. The stillbirth rate in mothers with poorly controlled hypertension is 9.4% (17), while good fetal outcomes have been observed in cases when blood pressure is well controlled. In most cases, if good blood pressure control can be achieved, surgical evaluation and management may be postponed until after delivery.
Spironolactone, a mineralocorticoid receptor antagonist (MRA), is the preferred treatment for PA in the non-pregnant population; however, spironolactone is a pregnancy risk category C medication, and is not recommended as first line therapy in pregnant women with PA. Animal studies have demonstrated feminization of the male fetus as well as decreased live birth rate (21) following spironolactone treatment. There have been case reports of women treated with spironolactone during pregnancy with healthy male infants delivered without malformations (22-24), although there is at least one case of neonatal genital ambiguity following spironolactone treatment (17). Eplerenone is another MRA that may be used in PA; however, animal studies demonstrate decreased fetal weight and increased post-implantation reabsorption. There is one case report of eplerenone used in PA during pregnancy without complications (25), but there have been no large human studies of eplerenone use during pregnancy.
Instead, amiloride, a potassium-sparing diuretic used in cases of MRA intolerance, is safe during pregnancy (26,27) and is thus the preferred agent (Table 1). There are several case reports of amiloride treatment in pregnant women with hyperaldosteronism as well as those with severe hypertension in pregnancy due to other etiologies (28,29) without negative effects on the fetus.

Full table
In cases where hypertension cannot be controlled medically, surgical treatment may be necessary. In these cases, MRI to evaluate for an adrenal adenoma is the preferred imaging study, although MRI cannot distinguish an aldosterone-producing adenoma from a non-functional (incidental) adenoma. If found, the best option is surgical removal of the adenoma in the second trimester (30). There are several case reports of adrenal adenoma removal in the literature leading to significant improvement in hypertension and hypokalemia. In six patients who underwent adrenalectomy, 5 had control of their hypertension, with an average term length at delivery of 33.7 wGA (17). In one patient who had a documented adrenal adenoma and resistant hypertension but did not undergo surgery, the infant was emergently delivered and did not survive (31).
CS
CS in pregnancy is rare as excessive cortisol interferes with the reproductive axis; however, CS can lead to significant maternal and fetal morbidity and mortality. While the most common cause of cortisol excess in the non-pregnant population is an adrenocorticotropic hormone (ACTH)-secreting pituitary adenoma [Cushing disease (CD)], CD is not frequently diagnosed during pregnancy, because ACTH-mediated hypersecretion of adrenal androgens leads to menstrual abnormalities. In pregnancy, almost 50% of CS is due to adrenal adenoma, 30% to CD, and 1–3% due to ectopic ACTH syndrome, which is typically associated with malignancy (32,33). In addition, 13% of CS is due to pregnancy-induced hypercortisolism, most commonly caused by abnormally high expression of the luteinizing hormone/chorionic gonadotropin (LH/CG) receptor on an adrenal adenoma or less commonly throughout the adrenal glands, which leads to cortisol excess when the elevated human CG of pregnancy stimulates these receptors (34-36).
Recognition of cortisol excess is crucial, as untreated CS during pregnancy has severe consequences. In untreated patients, 40.5% have hypertension, compared to 2.3% in patients with cured CS. Rates of diabetes mellitus (36.9%) and pre-eclampsia (26.3%) are similarly increased. Rarely, severe psychiatric complications, wound infections, osteoporosis, and heart failure may develop. The etiology of CS predicts the risk of fetal loss, with ectopic ACTH production and pregnancy-induced CS increasing the risk of fetal loss by 3 to 4 times that of CD. The preterm delivery rate in CD is 33%, and spontaneous abortion/still birth occurs in 17% of these pregnancies (33,37).
Clinical recognition of CS may be delayed during pregnancy due to the overlap between the signs and symptoms of cortisol excess and normal pregnancy. Typical CS symptoms of weight gain, striae, round facies, and glucose intolerance are common in pregnancy. More specific features of CS that are not common in pregnancy should prompt investigation. These include purple (not flesh-toned) striae greater than 1-cm wide, particularly if outside the abdomen, such as axilla and breast; proximal myopathy; ecchymoses; virilization; poor wound healing; and neuropsychiatric symptoms.
As with PA, diagnosis of CS can be difficult due to the changes in the hypothalamic-pituitary-adrenal (HPA) axis that occur during pregnancy. Hypothalamic corticotropin releasing hormone (CRH) stimulates pituitary ACTH production, which then drives cortisol secretion from the adrenal gland. Cortisol is then transported to the peripheral tissues bound to corticosteroid-binding globulin (CBG). Pregnancy results in alterations to each of these components.
During pregnancy, the placenta secretes CRH, leading to an increase in overall circulating CRH concentrations (38). ACTH rises during pregnancy as well, reaching a peak at delivery. Increased estrogen increases CBG glycosylation, preventing CBG cleavage and increasing concentrations two- to three-fold (39,40). This results in an increase in total serum cortisol levels (41). These changes affect the commonly used screening tests for CS (Figure 2).
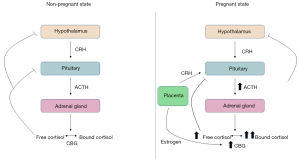
In the non-pregnant population, screening tests for CS include 24-hour urine free cortisol (UFC) measurement, late-night salivary cortisol measurement, and the dexamethasone suppression test (DST). The UFC has been used frequently in the literature and displays predictable changes during pregnancy. During the second and third trimester, UFC is typically increased 1.4–1.6 times, thus CS should not be diagnosed unless the UFC is at least 2–3 times the upper limit of normal (42).
Because saliva is an ultrafiltrate of plasma, salivary cortisol concentrations reflect plasma free cortisol values and avoid the influence of elevated CBG on serum total cortisol measurements. There are several case reports documenting the use of late-night salivary cortisol for diagnosis of CS during pregnancy (43,44); however, the cut-off values for diagnosis are controversial. Some reports suggest that salivary cortisol does not change significantly during pregnancy and that the normal adult levels may be used (45). Close examination of these studies reveals a large degree of variability in salivary cortisol levels in pregnant women, with some women without clinical hypercortisolism demonstrating elevated salivary cortisol. Using these data and similar studies, other authors have proposed normal values in pregnant women as <6.9 nmol/L in the first trimester, <7.2 nmol/L in the second trimester and <9.1 nmol/L in the third trimester, which results in a specificity between 80% to 92% (46).
Cortisol peaks in the early morning and drops in the late evening, and this circadian rhythm is maintained during the first and second trimesters of pregnancy but is blunted or lost in CS. Early studies have suggested performing AM and PM salivary cortisol measurements, with a 50% drop from morning to evening (41,47) to rule out CS; however, this approach has not been used in the more recent literature.
The DST measures serum cortisol levels after exposure to dexamethasone, which should suppress ACTH and result in low serum cortisol. In pregnant patients, the DST should be avoided as the elevation in CBG leads to increased serum cortisol levels irrespective of free cortisol levels, thus making incomplete cortisol suppression difficult to interpret. ACTH is also more difficult to suppress during pregnancy, perhaps due to placental CRH production, further complicating this dynamic test of adrenal function (48,49).
Once hypercortisolism has been diagnosed, determining the etiology is crucial to providing the appropriate treatment. CS is either ACTH-dependent (due to pituitary disease or ectopic ACTH expression) or ACTH-independent (typically adrenal in origin). Differentiating these two may also be challenging during pregnancy because placental CRH and ACTH can lead to elevated ACTH levels even in patients with adrenal CS (50). In general, ACTH greater than 35 pg/mL in the setting of an elevated cortisol suggests an ACTH-dependent source of cortisol excess, while an ACTH less than 5 pg/mL is consistent with adrenal CS (46,51). While there are several dynamic confirmatory tests that are typically used for non-pregnant patients, these have not been systematically studied during pregnancy and are not recommended.
In CS with elevated ACTH, the preferred imaging test is sella MRI. The pituitary gland increases in size during pregnancy, so this factor must be considered when evaluating pituitary MRI. Most corticotrope adenomas causing CD are small (<5 mm) and often do not distort the geometry of the pituitary. Instead, these tumors are typically identified due to their poor enhancement compared to the normal gland after gadolinium contrast administration. Unfortunately, gadolinium contrast must be avoided in the pregnant women, and the sensitivity of sella MRI for identifying CD tumors is therefore much lower (42,52). While inferior petrosal sinus sampling (IPSS) is used to confirm CD in the non-pregnant population, this is typically avoided during pregnancy due to excessive radiation exposure. There are reports of IPSS methods to minimize radiation to the fetus; however, fetal outcomes following these procedures have been poor (53). For adrenal CS, abdominal ultrasound and adrenal MRI without contrast are the preferred imaging studies (50).
Surgical management is a safe and effective option (50,54) for treatment of both pituitary and adrenal CS. Regardless of the procedure, surgery should be performed during the second trimester, preferably before the 24th week of gestation. Some reports of surgery performed during the third trimester have found an increased risk of prematurity (54-56) which might be due to either the procedure and/or the prolonged duration of hypercortisolism. Surgical strategies depend on the etiology of CS, with unilateral adrenalectomy preferred for adrenal adenoma. In cases of pregnancy-induced CS without a discreet adenoma, the most definitive treatment is bilateral adrenalectomy, which results in permanent adrenal insufficiency. Specific surgical considerations for these procedures in pregnant women have been reviewed elsewhere (57).
In cases where surgical management is not possible or must be delayed, medical treatment has been reported with variable success. The most commonly used medication to control CS during pregnancy is metyrapone (33). Metyrapone has been given during the first trimester prior to surgery (58) as well as throughout the entirety of pregnancy without birth defects (59,60). Use of metyrapone may be limited by cost and availability. In some instances, metyrapone was associated with increased blood pressure, presumably due to an increase in 11-deoxycorticosterone, a steroid intermediate which acts as a mineralocorticoid agonist (61). The use of other medications has only been reported in 1 to 2 cases each and include ketoconazole, mitotane and cabergoline (51). These drugs have typically been avoided due to teratogenicity. Mifepristone, a glucocorticoid antagonist used in CS in the non-pregnancy population, should be avoided due to its simultaneous progesterone antagonism, which is the basis for its use as an abortificant.
Treatment of CS results in improvements in maternal and fetal outcomes. The odds ratio for fetal loss was 0.25–0.34 for treated CS compared to women whose CS was not treated. Gestational diabetes, gestational hypertension, preeclampsia and Cesarean section rates in patients with active CS are significantly higher than in patients with cured CS, in whom the rates approach that of the healthy population (33). Due to the rarity of the condition and the variety of strategies used in the literature, there is no consensus as the best treatment strategy, although both surgical and medical options have been reported.
Pheochromocytoma and paraganglioma
Catecholamine excess can occur either due to pheochromocytoma, which arises from the chromaffin cells in the adrenal gland, or due to paraganglioma, which arises from sympathetic ganglia. The estimated incidence of pheochromocytoma and paraganglioma in pregnancy is 1 in 15,000 to 45,000; however, if left undiagnosed, the consequences can be devastating for both the mother and the fetus (30,62). Catecholamines are potent vasoconstrictors and increase cardiac contractility. Thus, if these tumors are not diagnosed before delivery, severe cardiovascular consequences might ensue. In early studies of pheochromocytoma (before the 1970s), the maternal and fetal death rates were 48% and 54%, respectively, if the tumor was not diagnosed before delivery. If diagnosed before term, the corresponding mortality rates decreased substantially to 5% and 15% (63-66). In more recent studies, if the tumor is diagnosed early, maternal survival is 100%, while the mortality rate is still 29% if left undiagnosed. Death is due to hypertensive crisis leading to acute coronary syndrome, cardiomyopathy, arrhythmias, stroke and shock (62).
Diagnosing pheochromocytomas and paragangliomas before delivery may be difficult due to the rarity of the disease and the presence of non-specific symptoms. Hypertension is often attributed to gestational hypertension or pre-eclampsia. Nearly 40% of patients with paraganglioma have an inherited genetic mutation, so a detailed family history can increase the index of suspicion (67). Pheochromocytoma is also often a part of genetic tumor syndromes. Other associated symptoms include paroxysmal hypertension, orthostatic hypotension, dizziness, palpitations and hypertension without ankle edema or proteinuria. Hypertension associated with pheochromocytoma or paraganglioma may be paroxysmal or constant, so the presence of persistent hypertension does not rule out the diagnosis.
During a healthy pregnancy, catecholamine levels are unchanged except at the onset of labor until the second post-partum day (68,69). Urinary catecholamines are increased 1.7- to 2.6-fold in hospitalized pre-eclamptic patients; however, plasma catecholamines tend to be decreased or normal (70-73). Metanephrine and normetanephrine are metabolites of epinephrine and norepinephrine, respectively, which are continuously produced from pheochromocytomas and paragangliomas and are the preferred diagnostic tests for these tumors in non-pregnant patients and during pregnancy as well. Either 24-hour urine metanephrines or plasma metanephrines can be used to establish the diagnosis. In patients for whom there is a high degree of suspicion, plasma metanephrines is preferred due to the excellent specificity of 97.5%. If negative, the diagnosis is effectively ruled out (62).
As with the non-pregnant population, false positive elevations in catecholamine and metanephrine measurements are common and may result from interfering medications or improperly collected samples (74). Labetalol and methyldopa, which are used frequently for hypertension in pregnancy, can lead to falsely elevated catecholamines and metanephrines when measured by older liquid chromatography assays; however, this interference is resolved by using tandem mass spectrometry (LC-MS/MS) assays (75). Some medications may also cause mild physiologic elevation in catecholamines and metanephrines, such as tricyclic antidepressants. Plasma catecholamines or metanephrines are best measured with the patient in the supine position, as being seated upright without prior supine rest can increase plasma metanephrines by as much as 30% (76). While provocative testing, such as the clonidine suppression test can be used in non-pregnant patients, it is contraindicated during pregnancy due to concern for adverse effects on the fetus.
Once a biochemical diagnosis of catecholamine excess due to a pheochromocytoma or paraganglioma is made, imaging is required for localization. Radionuclide imaging agents such as [123I]- or [131I]-metaiodobenzylguanidine (MIBG) or [68Ga]-DOTATATE are contra-indicated during pregnancy, and so ultrasound and MRI without contrast are the preferred imaging studies. While abdominal ultrasound is safe and accessible, it often misses small lesions, and visualization might be compromised by the gravid uterus in the third trimester. Unlike aldosterone-producing adenomas, symptomatic pheochromocytomas are typically several cm wide and have well-defined imaging characteristics, such as no signal loss on out-of-phase images and high signal on T2-weighted images (77,78). Consequently, MRI has sensitivity of 100% in lesions that are greater than 1.5 cm. Biopsy is contra-indicated due to concern that a catecholamine surge may initiate a hypertensive crisis.
Once the pheochromocytoma or paraganglioma has been identified, surgery is the most appropriate treatment. Surgical planning requires a multidisciplinary team, which includes experienced surgeons and anesthesiologists. Available studies suggest that surgical resection should occur before 24 weeks gestation or be delayed until the time of delivery. If surgery cannot be performed before 24 weeks, then the preferred delivery method is Cesarean section with concomitant tumor removal (66).
Prior to surgery, patients must undergo pre-surgical pharmacologic preparation to prevent cardiovascular collapse due to massive catecholamine release during surgery. The foundation of pre-surgical preparation is alpha-blockade with phenoxybenzamine or doxazosin. These medications have not been systematically evaluated in pregnant patients; however, the benefits of treatment outweigh the risks. When adequate alpha blockade is given, fetal mortality is decreased from 55% to 6%, and maternal mortality is decreased from 9.5% to 0% (79). Both phenoxybenzamine and doxazosin cross the placenta. Phenoxybenzamine, which unlike doxazosin irreversibly blocks the alpha receptors, has been associated with neonatal hypotension and respiratory distress, while doxazosin has not been associated with negative fetal outcomes (80,81). In addition to alpha-blockade, beta-blockade is often necessary to treat the tachycardia that may occur with alpha-blockade alone. During pregnancy, labetalol, which provides both alpha- and beta-blockade, can be used, although labetalol should not be started until several days after initiation of alpha-blocking agents (82). Patients should also receive aggressive hydration, as patients with pheochromocytoma and paragangliomas are volume-contracted and may have orthostatic hypotension, which alpha-blockade can exacerbate.
Conclusions
Endocrine causes of hypertension during pregnancy are rare, but these conditions are important to recognize and treat aggressively to avoid harm to the mother and fetus. PA, although common in the non-pregnant population, is very rarely reported during pregnancy. Adequate blood pressure control, often achieved medically, is the most important factor in predicting pregnancy outcomes. Complete evaluation including confirmatory testing and localization can be delayed until after delivery. In contrast, CS during pregnancy carries a very high rate of morbidity and mortality if it is managed symptomatically. Instead, surgical intervention is preferred. Metyrapone to manage hypercortisolemia may be used prior to surgery or in cases where surgery must be delayed. Pheochromocytoma and paraganglioma have the highest rate of maternal and fetal mortality if not discovered prior to delivery. Surgery with adequate medical preparation prior to 24 weeks’ gestation or coordinated with Cesarean section if not discovered earlier can decrease the maternal mortality rate to 0%.
Acknowledgments
Funding: Alison H. Affinati received support from T32DK007245 and F32DK122660.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fardella CE, Mosso L, Gómez-Sánchez C, et al. Primary hyperaldosteronism in essential hypertensives: prevalence, biochemical profile, and molecular biology. J Clin Endocrinol Metab 2000;85:1863-7. [PubMed]
- Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 2006;48:2293-300. [Crossref] [PubMed]
- Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 2004;89:1045-50. [Crossref] [PubMed]
- Morton A. Primary aldosteronism and pregnancy. Pregnancy Hypertens 2015;5:259-62. [Crossref] [PubMed]
- Young WF Jr. Chapter 16 - Endocrine Hypertension. In: Melmed S, Polonsky KS, Larsen PR, et al. editors. Williams Textbook of Endocrinology. 17th Edition. Philadelphia: Elsevier, 2015:556-88.
- Berga SL, Nitsche JF, Braunstein GD. Chapter 21 - Endocrine Changes in Pregnancy. In: Melmed S, Polonsky KS, Larsen PR, et al. editors. Williams Textbook of Endocrinology. 17th Edition. Philadelphia: Elsevier, 2016:831-48.
- Franks RC, Hayashi RH. Maternal and fetal renin activity and renin and big renin concentrations in second-trimester pregnancy. Am J Obstet Gynecol 1979;134:20-2. [Crossref] [PubMed]
- Keely E. Endocrine causes of hypertension in pregnancy--when to start looking for zebras. Semin Perinatol 1998;22:471-84. [Crossref] [PubMed]
- Wilson M, Morganti AA, Zervoudakis I, et al. Blood pressure, the renin-aldosterone system and sex steroids throughout normal pregnancy. Am J Med 1980;68:97-104. [Crossref] [PubMed]
- Pirani BB, Campbell DM, MacGillivray I. Plasma volume in normal first pregnancy. J Obstet Gynaecol Br Commonw 1973;80:884-7. [Crossref] [PubMed]
- Ronconi V, Turchi F, Zennaro MC, et al. Progesterone increase counteracts aldosterone action in a pregnant woman with primary aldosteronism. Clin Endocrinol (Oxf) 2011;74:278-9. [Crossref] [PubMed]
- Gordon RD, Tunny TJ. Aldosterone-producing-adenoma (A-P-A): effect of pregnancy. Clin Exp Hypertens A 1982;4:1685-93. [Crossref] [PubMed]
- Okawa T, Asano K, Hashimoto T, et al. Diagnosis and management of primary aldosteronism in pregnancy: case report and review of the literature. Am J Perinatol 2002;19:31-6. [Crossref] [PubMed]
- Eguchi K, Hoshide S, Nagashima S, et al. An adverse pregnancy-associated outcome due to overlooked primary aldosteronism. Intern Med 2014;53:2499-504. [Crossref] [PubMed]
- Albiger NM, Sartorato P, Mariniello B, et al. A case of primary aldosteronism in pregnancy: do LH and GNRH receptors have a potential role in regulating aldosterone secretion? Eur J Endocrinol 2011;164:405-12. [Crossref] [PubMed]
- Shigematsu K, Yamaguchi N, Nakagaki T, et al. A case of unilateral adrenal hyperplasia being difficult to distinguish from aldosterone-producing adenoma. Exp Clin Endocrinol Diabetes 2009;117:124-8. [Crossref] [PubMed]
- Landau E, Amar L. Primary aldosteronism and pregnancy. Ann Endocrinol (Paris) 2016;77:148-60. [Crossref] [PubMed]
- Wyckoff JA, Seely EW, Hurwitz S, et al. Glucocorticoid-remediable aldosteronism and pregnancy. Hypertension 2000;35:668-72. [Crossref] [PubMed]
- Malha L, August P. Secondary Hypertension in Pregnancy. Curr Hypertens Rep 2015;17:53. [Crossref] [PubMed]
- Funder JW, Carey RM, Mantero F, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016;101:1889-916. [Crossref] [PubMed]
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol 1980;95:540-5. [Crossref] [PubMed]
- Neerhof MG, Shlossman PA, Poll DS, et al. Idiopathic aldosteronism in pregnancy. Obstet Gynecol 1991;78:489-91. [PubMed]
- Lotgering FK, Derkx FM, Wallenburg HC. Primary hyperaldosteronism in pregnancy. Am J Obstet Gynecol 1986;155:986-8. [Crossref] [PubMed]
- Riester A, Reincke M. Progress in primary aldosteronism: mineralocorticoid receptor antagonists and management of primary aldosteronism in pregnancy. Eur J Endocrinol 2015;172:R23-30. [Crossref] [PubMed]
- Cabassi A, Rocco R, Berretta R, et al. Eplerenone use in primary aldosteronism during pregnancy. Hypertension 2012;59:e18-19. [Crossref] [PubMed]
- Griffing GT, Aurecchia SA, Sindler BH, et al. The effect of amiloride on the renin-aldosterone system in primary hyperaldosteronism and Bartter’s syndrome. J Clin Pharmacol 1982;22:505-12. [Crossref] [PubMed]
- Izzo JL, Hong M, Hussain T, et al. Maintenance of long-term blood pressure control and vascular health by low-dose amiloride-based therapy in hyperaldosteronism. J Clin Hypertens (Greenwich) 2019;21:1183-90. [Crossref] [PubMed]
- Krysiak R, Samborek M, Stojko R. Primary aldosteronism in pregnancy. Acta Clin Belg 2012;67:130-4. [PubMed]
- Al-Ali NA, El-Sandabesee D, Steel SA, et al. Conn’s syndrome in pregnancy successfully treated with amiloride. J Obstet Gynaecol 2007;27:730-1. [Crossref] [PubMed]
- Kamoun M, Mnif MF, Charfi N, et al. Adrenal diseases during pregnancy: pathophysiology, diagnosis and management strategies. Am J Med Sci 2014;347:64-73. [Crossref] [PubMed]
- Kreze A, Kothaj P, Dobáková M, et al. Primary aldosteronism caused by aldosterone-producing adenoma in pregnancy--complicated by EPH gestosis. Wien Klin Wochenschr 1999;111:855-7. [PubMed]
- Buescher MA, McClamrock HD, Adashi EY. Cushing syndrome in pregnancy. Obstet Gynecol 1992;79:130-7. [PubMed]
- Caimari F, Valassi E, Garbayo P, et al. Cushing’s syndrome and pregnancy outcomes: a systematic review of published cases. Endocrine 2017;55:555-63. [Crossref] [PubMed]
- Achong N, D’Emden M, Fagermo N, et al. Pregnancy-induced Cushing’s syndrome in recurrent pregnancies: case report and literature review. Aust N Z J Obstet Gynaecol 2012;52:96-100. [Crossref] [PubMed]
- Rask E, Schvarcz E, Hellman P, et al. Adrenocorticotropin-independent Cushing’s syndrome in pregnancy related to overexpression of adrenal luteinizing hormone/human chorionic gonadotropin receptors. J Endocrinol Invest 2009;32:313-6. [Crossref] [PubMed]
- Chui MH, Ozbey NC, Ezzat S, et al. Case report: Adrenal LH/hCG receptor overexpression and gene amplification causing pregnancy-induced Cushing's syndrome. Endocr Pathol 2009;20:256-61. [Crossref] [PubMed]
- Pickard J, Jochen AL, Sadur CN, et al. Cushing’s syndrome in pregnancy. Obstet Gynecol Surv 1990;45:87-93. [Crossref] [PubMed]
- Goland RS, Wardlaw SL, Stark RI, et al. High levels of corticotropin-releasing hormone immunoactivity in maternal and fetal plasma during pregnancy. J Clin Endocrinol Metab 1986;63:1199-203. [Crossref] [PubMed]
- Mitchell E, Torpy DJ, Bagley CJ. Pregnancy-associated Corticosteroid-binding Globulin: High Resolution Separation of Glycan Isoforms. Horm Metab Res 2004;36:357-9. [Crossref] [PubMed]
- Nenke MA, Zeng A, Meyer EJ, et al. Differential Effects of Estrogen on Corticosteroid-Binding Globulin Forms Suggests Reduced Cleavage in Pregnancy. J Endocr Soc 2017;1:202-10. [PubMed]
- Nolten WE, Lindheimer MD, Rueckert PA, et al. Diurnal patterns and regulation of cortisol secretion in pregnancy. J Clin Endocrinol Metab 1980;51:466-72. [Crossref] [PubMed]
- Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008;93:1526-40. [Crossref] [PubMed]
- Mellor A, Harvey RD, Pobereskin LH, et al. Cushing’s disease treated by trans-sphenoidal selective adenomectomy in mid-pregnancy. Br J Anaesth 1998;80:850-2. [Crossref] [PubMed]
- Doshi S, Bhat A, Lim KB. Cushing’s syndrome in pregnancy. J Obstet Gynaecol 2003;23:568-9. [Crossref] [PubMed]
- Ambroziak U, Kondracka A, Bartoszewicz Z, et al. The morning and late-night salivary cortisol ranges for healthy women may be used in pregnancy. Clin Endocrinol (Oxf) 2015;83:774-8. [Crossref] [PubMed]
- Lopes LML, Francisco RPV, Galletta MAK, et al. Determination of nighttime salivary cortisol during pregnancy: comparison with values in non-pregnancy and Cushing’s disease. Pituitary 2016;19:30-8. [Crossref] [PubMed]
- Streeten DH, Stevenson CT, Dalakos TG, et al. The diagnosis of hypercortisolism. Biochemical criteria differentiating patients from lean and obese normal subjects and from females on oral contraceptives. J Clin Endocrinol Metab 1969;29:1191-211. [Crossref] [PubMed]
- Cousins L, Rigg L, Hollingsworth D, et al. Qualitative and quantitative assessment of the circadian rhythm of cortisol in pregnancy. Am J Obstet Gynecol 1983;145:411-6. [Crossref] [PubMed]
- Rees LH, Burke CW, Chard T, et al. Possible placental origin of ACTH in normal human pregnancy. Nature 1975;254:620-2. [Crossref] [PubMed]
- Lindsay JR, Jonklaas J, Oldfield EH, et al. Cushing’s syndrome during pregnancy: personal experience and review of the literature. J Clin Endocrinol Metab 2005;90:3077-83. [Crossref] [PubMed]
- Brue T, Amodru V, Castinetti F. MANAGEMENT OF ENDOCRINE DISEASE: Management of Cushing’s syndrome during pregnancy: solved and unsolved questions. Eur J Endocrinol 2018;178:R259-66. [Crossref] [PubMed]
- Tabarin A, Laurent F, Catargi B, et al. Comparative evaluation of conventional and dynamic magnetic resonance imaging of the pituitary gland for the diagnosis of Cushing’s disease. Clin Endocrinol (Oxf) 1998;49:293-300. [Crossref] [PubMed]
- Pinette MG, Pan YQ, Oppenheim D, et al. Bilateral inferior petrosal sinus corticotropin sampling with corticotropin-releasing hormone stimulation in a pregnant patient with Cushing’s syndrome. Am J Obstet Gynecol 1994;171:563-4. [Crossref] [PubMed]
- Sam S, Molitch ME. Timing and special concerns regarding endocrine surgery during pregnancy. Endocrinol Metab Clin North Am 2003;32:337-54. [Crossref] [PubMed]
- Jolly K, Darr A, Arlt W, et al. Surgery for Cushing's disease in pregnancy: our experience and a literature review. Ann R Coll Surg Engl 2019;101:e26-31. [Crossref] [PubMed]
- Sammour RN, Saiegh L, Matter I, et al. Adrenalectomy for adrenocortical adenoma causing Cushing’s syndrome in pregnancy: a case report and review of literature. Eur J Obstet Gynecol Reprod Biol 2012;165:1-7. [Crossref] [PubMed]
- Guidelines for the Use of Laparoscopy during Pregnancy - A SAGES Publication. SAGES. (cited 2019 Oct 01). Available online: https://www.sages.org/publications/guidelines/guidelines-for-diagnosis-treatment-and-use-of-laparoscopy-for-surgical-problems-during-pregnancy/
- Blanco C, Maqueda E, Rubio JA, et al. Cushing’s syndrome during pregnancy secondary to adrenal adenoma: metyrapone treatment and laparoscopic adrenalectomy. J Endocrinol Invest 2006;29:164-7. [Crossref] [PubMed]
- Close CF, Mann MC, Watts JF, et al. ACTH-independent Cushing’s syndrome in pregnancy with spontaneous resolution after delivery: control of the hypercortisolism with metyrapone. Clin Endocrinol (Oxf) 1993;39:375-9. [Crossref] [PubMed]
- Gormley MJ, Hadden DR, Kennedy TL, et al. Cushing’s syndrome in pregnancy--treatment with metyrapone. Clin Endocrinol (Oxf) 1982;16:283-93. [Crossref] [PubMed]
- Connell JMC, Cordiner J, Davies DL, et al. Pregnancy complicated by Cushing’s syndrome: potential hazard of metyrapone therapy. Case report. Br J Obstet Gynaecol 1985;92:1192-5. [Crossref] [PubMed]
- Lenders JWM, Langton K, Langenhuijsen JF, et al. Pheochromocytoma and Pregnancy. Endocrinol Metab Clin North Am 2019;48:605-17. [Crossref] [PubMed]
- Harper MA, Murnaghan GA, Kennedy L, et al. Phaeochromocytoma in pregnancy. Five cases and a review of the literature. Br J Obstet Gynaecol 1989;96:594-606. [Crossref] [PubMed]
- Ahlawat SK, Jain S, Kumari S, et al. Pheochromocytoma associated with pregnancy: case report and review of the literature. Obstet Gynecol Surv 1999;54:728-37. [Crossref] [PubMed]
- Schenker JG, Granat M. Phaeochromocytoma and pregnancy--an updated appraisal. Aust N Z J Obstet Gynaecol 1982;22:1-10. [Crossref] [PubMed]
- Biggar MA, Lennard TWJ. Systematic review of phaeochromocytoma in pregnancy. Br J Surg 2013;100:182-90. [Crossref] [PubMed]
- Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol 2015;11:101-11. [Crossref] [PubMed]
- Zuspan FP. Urinary excretion of epinephrine and norepinephrine during pregnancy. J Clin Endocrinol Metab 1970;30:357-60. [Crossref] [PubMed]
- Goodall M, Diddle AW. Epinephrine and norepinephrine in pregnancy. A comparative study of the adrenal gland and catechol output in different species of animals and man. Am J Obstet Gynecol 1971;111:896-904. [Crossref] [PubMed]
- Zuspan FP. Catecholamines. Their role in pregnancy and the development of pregnancy-induced hypertension. J Reprod Med 1979;23:143-50. [PubMed]
- Zuspan FP. Adrenal gland and sympathetic nervous system response in eclampsia. Am J Obstet Gynecol 1972;114:304-13. [Crossref] [PubMed]
- Pedersen EB, Rasmussen AB, Christensen NJ, et al. Plasma noradrenaline and adrenaline in pre-eclampsia, essential hypertension in pregnancy and normotensive pregnant control subjects. Acta Endocrinol 1982;99:594-600. [Crossref] [PubMed]
- Rubin PC, Butters L, McCabe R, et al. Plasma catecholamines in pregnancy induced hypertension. Clin Sci 1986;71:111-5. [Crossref] [PubMed]
- Yu R, Wei M. False positive test results for pheochromocytoma from 2000 to 2008. Exp Clin Endocrinol Diabetes 2010;118:577-85. [Crossref] [PubMed]
- Eisenhofer G, Peitzsch M. Laboratory evaluation of pheochromocytoma and paraganglioma. Clin Chem 2014;60:1486-99. [Crossref] [PubMed]
- Lenders JWM, Willemsen JJ, Eisenhofer G, et al. Is supine rest necessary before blood sampling for plasma metanephrines? Clin Chem 2007;53:352-4. [Crossref] [PubMed]
- Newhouse JH. MRI of the adrenal gland. Urol Radiol 1990;12:1-6. [Crossref] [PubMed]
- Velchik MG, Alavi A, Kressel HY, et al. Localization of pheochromocytoma: MIBG [correction of MIGB], CT, and MRI correlation. J Nucl Med 1989;30:328-36. [PubMed]
- Burgess GE. Alpha blockade and surgical intervention of pheochromocytoma in pregnancy. Obstet Gynecol 1979;53:266-70. [PubMed]
- Aplin SC, Yee KF, Cole MJ. Neonatal effects of long-term maternal phenoxybenzamine therapy. Anesthesiology 2004;100:1608-10. [Crossref] [PubMed]
- Versmissen J, Koch BCP, Roofthooft DWE, et al. Doxazosin treatment of phaeochromocytoma during pregnancy: placental transfer and disposition in breast milk. Br J Clin Pharmacol 2016;82:568-9. [Crossref] [PubMed]
- Lenders JWM, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2014;99:1915-42. [Crossref] [PubMed]


