Oncoplastic breast surgery for centrally located breast cancer: a case series
Introduction
Oncoplastic breast surgery (OBS), which combines the concepts of oncologic and plastic surgery, is becoming more common, especially in Western countries (1-6). At present, there are many different oncoplastic surgical techniques such as careful planning of skin and parenchymal excisions, reshaping of the gland following parenchymal excisions, and repositioning of the nipple areola complex (NAC) to the center of the breast mound with or without a correction in the contralateral breast to achieve better symmetry (7). The concept of OBS combining partial mastectomy with the breast reduction technique has become more popular; however, few studies have been conducted by Japanese institutions (8-11).
We previously reported that resection of partial deformities followed by immediate volume replacement using a local flap or distant autologous graft resulted in good outcomes for patients with non-ptotic breasts (12-19).
We herein report OBS performed on patients with centrally located breast cancer (CLBC) or Paget’s disease in our institution. Immediate breast reshaping using a latissimus dorsi mini flap (20), free dermal fat graft (FDFG) (15-17,19), and key hole-shaped skin glandular flap (21) were selected for patients with non-ptotic breasts, whereas OBS using the Grisotti flap (22,23) and amputation and free-NAC grafting (24) were selected for patients with CLBC in ptotic and/or large breasts, respectively.
Patients and diagnosis
Four Japanese patients were diagnosed with Paget’s disease (cases 3, 4, 5, and 6), one with centrally located invasive ductal carcinoma (IDC) (case 7), and three with ductal carcinoma and ductal spread to the nipple (cases 1, 2, and 8) in our institution between February 2006 and April 2012. None of these patients received preoperative systemic chemotherapy or endocrine therapy. They were examined preoperatively and diagnosed with adequate disease for breast conserving surgery by mammography (MMG), ultrasonography (US), computed tomography (CT), and magnetic resonance image (MRI) systems and histological findings. According to the spread of intramammary lesions and the distance between the lesions and the NAC, we preoperatively decided whether the NAC should be resected or not. Sentinel lymph node (SLN) biopsy using the radioisotope (RI) and dye method was performed to avoid axillary lymphadenectomy in six patients, while axillary lymphadenectomy was performed on two patients with IDC (cases 1 and 7) according to the indication for axillary preservation at that time. Neither local nor distant recurrence was observed in any patients within a median observation period of 46.6 months.
Case presentation
Latissimus dorsi mini flap (case 1) (20)
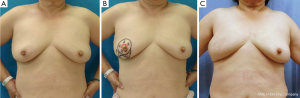
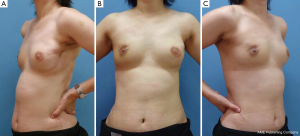
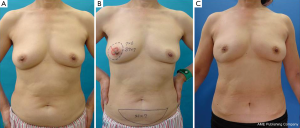
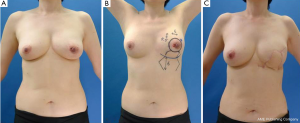
A 37-year-old patient with a slim body and non-ptotic breasts was diagnosed with a T1 cancerous tumor on the upper-outer quadrant area of the left breast. She wanted to undergo partial mastectomy and immediate volume replacement using autologous tissue, but not an implant. We planned, designed, and drew the resected area and latissimus dorsi myocutaneous flap (case 1, Figure 1). During surgery, several margins were examined histologically and revealed to be cancer-free; however, the edge toward the NAC was positive for ductal spread. The NAC was completely removed (Figure 2A) and the latissimus dorsi flap with ellipse-shaped skin was raised and passed from an anatomically normal position toward the anterior deformity via an incision line on the anterior axillary line (Figure 2A,B). The latissimus dorsi flap was sutured to some edges of the remnant gland following trimming such as rolling and gathering until the treated breast shape was the same as that of the contralateral breast (Figure 2C). The deformity after removal of the NAC was restored by trimming the skin in a circle (Figure 2D). Five years after surgery, she was diagnosed with primary breast cancer in the contralateral right breast (Figure 3A). We performed partial mastectomy and immediate volume replacement using a FDFG from the lateral abdomen according to a previous report (18). The NAC for the left breast was reconstructed during surgery (Figure 3B,C). Free grafts from the bilateral groins and half of the nipple from the right breast, following an intraoperative histological examination to confirm that this tissue was cancer-free, were used to reconstruct the left NAC (Figure 4). The nipple graft bolster was removed on postoperative day 7. Excellent symmetry was obtained six months after radiation therapy to the right breast (Figure 5).
Free dermal fat graft (FDFG) (cases 2 and 3) (15-17)
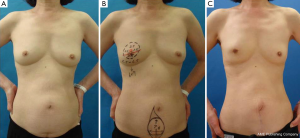
A 57-year-old Japanese female had an operation scar on her lower abdomen in a cranio-caudal direction due to a gynecological disorder (case 2, Figure 6A). A histological examination of erosion on the right nipple revealed that she has Paget’s disease in her right breast. Partial mastectomy with the whole nipple, but not the areola via a horizontal incision and SLN biopsy via another incision were performed. A cylinder-shaped piece of breast tissue was removed and the partial defect on the central area of the breast was repaired using a FDFG from the lower abdomen (15,16) (Figure 6B). Although she did not want to reconstruct the right nipple, the cosmetic results achieved were excellent four years after surgery (Figure 6C).
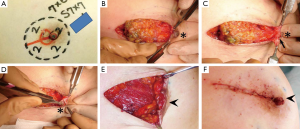
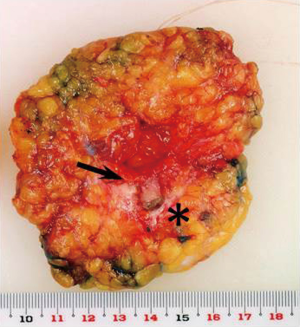
A 58-year-old patient was diagnosed with Paget’s disease with an intraductal component restricted to just under the central area of the right breast (case 3). A 3-mm erosive lesion was found in the center of the right nipple (Figure 7). Complete resection of the top and internal ductal component of the nipple, but not of the areola or lateral surface of the nipple was planned (Figures 7B,8A-D). Immediate volume replacement using a FDFG from the lower abdomen followed by repair of the left nipple were performed. Lateral colored skin following resection of the ductal component was sutured, resulting in a smaller nipple than the original one (Figure 8E,F). The Paget’s lesion and ductal spread via the nipple to the breast tissue were completely resected, and 60 g of breast tissue with the nipple was resected (Figure 9). After in situ de-epithelialization, sharp dissection, and trimming, 80 g of the FDFG was implanted into the defect with the dermis facing the surface of the pectoralis major muscle (15). The dermis of the FDFG was peripherally sutured to the edge of the fascia of the major pectoral muscle using 3-0 Vicryl sutures. Good symmetry was maintained seven years after surgery; however, the operated nipple was smaller than that of the contralateral healthy one (Figure 7C).
Key hole-shaped skin glandular flap for patients with non-ptotic breasts (cases 4 and 5) (18)
A 65-year-old Japanese patient with non-ptotic breasts was diagnosed with Paget’s disease in her left breast. Preoperative US, CT, and MRI showed that intraductal spread to the breast tissue was restricted to just under the areola (case 4). Both breasts were placed into the operative field to allow the surgeon to observe the size, projection, and level of the inframammary line of the healthy contralateral breast for the maintenance of symmetry. We removed a cylinder-shaped piece of gland with surface and bottom circles of 50 and 55 mm in diameter, and 15 mm of normal skin around the cancerous lesion on the NAC, which was 23 mm × 21 mm in diameter. The fascia of the major pectoral muscle was removed at the same time. Several surgical margins were histologically examined during the operation to ensure that the cancerous lesions were completely removed. A keyhole-shaped skin glandular flap was raised according to the marked lines (Figure 10). Perforators were reserved as much as possible. An inframammary line was drawn on the parenchymal tissue containing the perforator and fascia of the anterior serratus muscle to maintain symmetry with the contralateral healthy breast. We moved the flap to the cranial side. A new inframammary line was drawn on the surface of the skin located at the bottom of the keyhole. Suturing with 3-0 Vicryl fixed the two lines drawn on the parenchymal tissue and skin as an inframammary line, and a new inframammary fold reappeared.
The postoperative findings one year after surgery of case 5 without ptotic breasts were shown in Figure 11.
Grisotti flap (cases 6 and 7) (22,23)
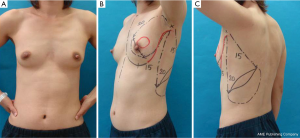
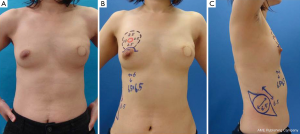
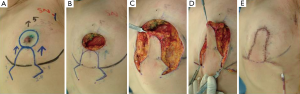
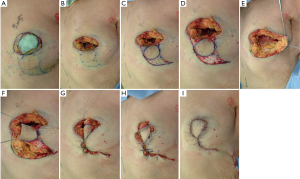
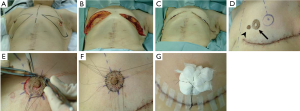
An 82-year-old patient with ptotic breasts was diagnosed with Paget’s disease in her left breast (case 6). A preoperative study using MMG, US, histological examinations (core needle biopsy or wedge biopsy of nipple erosion), bone scintigraphy, and MRI was performed. The cancerous lesion had both an erosive lesion on the NAC and intraductal components restricted to the retroareolar area (Figure 12A). With the patient in a standing position, we ensured that the nipple was located below the inframammary line and the softness and amount of skin was sufficient enough for the Grisotti flap. With the patient in a supine position, the edge of the tumor was determined by US and marks were placed on the skin surface. The partial mastectomy line was then marked in the form of a circular line using permanent ink 2 cm beyond the area of skin erosion. A curvilinear flap and neonipple line were marked on the breast with the patient in a standing position (Figure 12B,C).
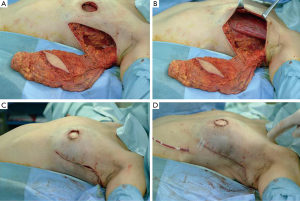
The cylinder-shaped piece of gland and NAC with surgical margins were removed (Figure 13A,B). During the operation, several surgical margins were histologically examined to ensure that the cancerous lesion was completely removed. A curvilinear flap was obtained inferior to the defect (Figure 13C). The flap was then de-epithelialized, except for a circular area of skin close to the defect, which was lifted intact in order to form the neonipple, with blood being supplied from a lateral pedicle (Figure 13D-F). The flap was incised medially and along the inframammary fold down to the pectoralis fascia, before being undermined laterally from the fascia to allow rotation and advancement of the flap to fill the defect. The skin-glandular flap was then rotated into the central quadrantectomy defect, and its deep part was sutured to the deep aspect of the breast defect with two to three 3-0 Vicryl sutures to fill the empty space around the defect and ensure adequate projection to the tip of the breast mound (Figure 13G-I). The tumor was removed, with a favorable esthetic outcome (Figure 12C).
We performed partial mastectomy and immediate breast reshaping using the Grisotti method on an invasive lesion just under the right NAC and axillary lymphadenectomy in case 7. Five years after surgery, excellent symmetry was obtained even though the patient had no NAC (case 7, Figure 14).
Amputation and NAC grafting for ptotic breasts (case 8) (24)
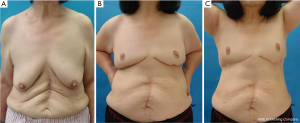
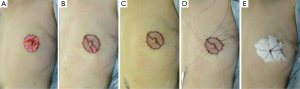
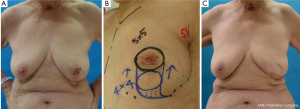
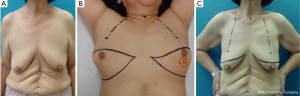
A 65-year-old Japanese woman with a past history of abdominal surgery was referred to us for an investigation of grouped calcification on the MMG of her left breast. US and MRI revealed ductal carcinoma in situ, which was restricted to the lower quadrant of the left breast, but was positive for ductal spread toward the left nipple. Oncologically, partial mastectomy of the left breast together with the left nipple was possible; however, difficulties achieving a good symmetrical outcome were anticipated due to the degree of ptosis. We decided on OBS combining amputation and NAC grafting (25,26). The incision line was drawn in black (Figure 15). We performed partial mastectomy with SLN biopsy. Excessive skin and parenchymal tissue, including the nipple, were removed with the fasciae of the pectoralis major muscle and serratus muscle. An intraoperative histological examination revealed that the three surgical margins were cancer-free and negative for metastasis in one SLN (Figure 16A-C). The healthy nipple of the right breast was taken and divided into two pieces for the new nipples (Figure 16D). The bilateral areolas were preserved prior to amputation of the breasts. After marking the NAC site, it was de-epithelialized, and a piece of the right nipple was sutured to the center of the de-epithelialized NAC site (Figure 16E). The free areola graft was then sutured to the site with interrupted and circumareolar sutures, before 4-0 Nylon bolster ties were sutured through the graft and the skin edge at eight circumferential points. A tie-over bolster of gauze and cotton was secured with 4-0 silk bolster ties (Figure 16F,G).
The nipple position, size, and degree of projection of the bilateral nipple were symmetrical four years after surgery (Figure 17).
Discussion
Patients with CLBC account for 5% to 20% of breast cancer cases and, for a long time, they have been denied breast conservation surgery (BCS) and instead been conventionally treated with mastectomy (27). The high incidence of NAC involvement associated with these tumors necessitates nipple and areola resection together with an adequate safety margin around the tumor, which has yielded acceptable cosmetic results and oncological control (28). Although Paget’s disease of the nipple has been extensively studied, its optimal treatment remains the subject of controversy. In 1991, Dixon et al. (29) reported the results obtained from 48 cases of Paget’s disease without a palpable lump that had undergone either simple mastectomy or cone excision of the NAC. They condemned conservation surgery in these cases because locoregional recurrence was found in 40% of cases after cone excision and in only 5.4% of cases after mastectomy. On the other hand, other reports concluded that BCT could safety be proposed to patients with Paget’s disease (14,29-32). Simple closure of the central defect, both vertically and horizontally, gave the breast a particular shape, which appeared as if the breast had been amputated at the tip (23). Central quadrantectomy including NAC resection through an elliptical incision was advocated by Pezzi et al. (33) and yielded satisfactory results without reconstruction. Clough et al. (34) advocated immediate breast repair after central tumor resection, and declared that cosmetic results were poor following simple lumpectomy and that secondary reconstruction in the breast was very difficult.
We performed OBS involving partial mastectomy with immediate breast reshaping using volume replacement or volume displacement for Paget’s disease or CLBC in Japanese patients in this series. According to previous reports, we selected OBS based on the size and shape of the breast in the standing position; volume replacement for patients with non-ptotic breasts, volume displacement using a skin-glandular flap or amputation for patients with ptotic breasts. All procedures were successfully performed both cosmetically and oncologically.
The number of cases in this series was not large, and the follow-up period was short; however, we demonstrated that OBS for Paget’s disease or CLBC produced good cosmetic results in Asian as well as in Western females.
Conclusions
Oncoplastic surgery combining partial mastectomy with immediate breast reshaping using a keyhole-shaped skin glandular flap was successfully performed in patients with Paget’s disease. Partial mastectomy, but not total mastectomy or immediate breast reconstruction can be selected. This surgery is expected to become more popular for the treatment of patients without large and ptotic breasts.
Acknowledgements
Disclosure: We declare no financial relationships or other interests associated with this manuscript, which might be construed as constituting a conflict of interest. We’ve stated that the material has not been previously published or submitted elsewhere for publication.
References
- Audretsch WP, Rezai M, Kolotas C, et al. Onco-plastic surgery: “target” volume reduction (BCT-mastopexy), lumpectomy reconstruction (BCT-reconstruction) and flap-supported operability in breast cancer. In: Proceedings of the 2nd European Congress on Senology; October 1994; Vienna, Austria; Moncuzzi, Bologna, Italy, 139-57.
- Audretsch WP, Rezai M, Kolotas C, et al. Tumor-specific immediate reconstruction (TSIR) in breast cancer patients. Perspect Plast Surg 1998;11:71-106.
- Clough KB, Cuminet J, Fitoussi A, et al. Cosmetic sequelae after conservative treatment for breast cancer: classification and results of surgical correction. Ann Plast Surg 1998;41:471-81. [PubMed]
- Bostwick J 3rd, Paletta C, Hartrampf CR. Conservative treatment for breast cancer. complications requiring reconstructive surgery. Ann Surg 1986;203:481-90. [PubMed]
- Petit JY, Rietjens M. Deormities following tumorectomy and partial mastectomy. In: Noon B. eds. Plastic and reconstructive surgery of the breast. Philadelphia: Marcel Decker, 1991:455-66.
- Clough KB, Nos C, Salmon RJ, et al. Conservative treatment of breast cancers by mammaplasty and irradiation: a new approach to lower quadrant tumors. Plast Reconstr Surg 1995;96:363-70. [PubMed]
- Masetti R, Pirulli PG, Magno S, et al. Oncoplastic techniques in the conservative surgical treatment of breast cancer. Breast Cancer 2000;7:276-80. [PubMed]
- Kijima Y, Yoshinaka H, Funasako Y, et al. Oncoplastic surgery after mammary reduction and mastopexy for bilateral breast cancer lesions: report of a case. Surg Today 2008;38:335-9. [PubMed]
- Kijima Y, Yoshinaka H, Ishigami S, et al. Oncoplastic surgery for Japanese patients with ptotic breasts. Breast Cancer 2011;18:273-81. [PubMed]
- Kijima Y, Yoshinaka H, Hirata M, et al. Oncoplastic surgery for Japanese patients with breast cancer of the lower pole. Surg Today 2011;41:1461-5. [PubMed]
- Zaha H, Hakazu O, Watanabe M, et al. Breast-conserving surgery using reduction mammoplasty. Jpn J Breast Cancer 2008;23:211-5.
- Kijima Y, Yoshinaka H, Funasako Y, et al. Immediate reconstruction using thoracodorsal adipofascial flap after partial mastectomy. Breast 2009;18:126-9. [PubMed]
- Kijima Y, Yoshinaka H, Owaki T, et al. Immediate reconstruction using inframammary adipofascial flap of the anterior rectus sheath after partial mastectomy. Am J Surg 2007;193:789-91. [PubMed]
- Kijima Y, Yoshinaka H, Hirata M, et al. Immediate reconstruction using a modified inframammary adipofascial flap after partial mastectomy. Surg Today 2013;43:456-60. [PubMed]
- Kijima Y, Yoshinaka H, Owaki T, et al. Early experience of immediate reconstruction using autologous free dermal fat graft after breast conservational surgery. J Plast Reconstr Aesthet Surg 2007;60:495-502. [PubMed]
- Kijima Y, Yoshinaka H, Hirata M, et al. Clinical and pathologic evaluation of implanted free dermal fat grafts after breast cancer surgery: a retrospective analysis. Surgery 2012;151:444-55. [PubMed]
- Kijima Y, Yoshinaka H, Funasako Y, et al. Immediate breast reconstruction using autologous free dermal fat grafts provides better cosmetic results for patients with upper inner cancerous lesions. Surg Today 2011;41:477-89. [PubMed]
- Kijima Y, Yoshinaka H, Hirata M, et al. Oncoplastic breast surgery combining partial mastectomy with immediate breast reshaping using a keyhole-shaped skin glandular flap for Paget’s disease. Surg Today 2013. [Epub ahead of print]. [PubMed]
- Kijima Y, Yoshinaka H, Hirata M, et al. Immediate volume replacement using modified free dermal fat graft from lateral abdomen for a patient with early breast cancer. Int Canc Conf J 2013;2:101-6.
- Noguchi M, Taniya T, Miyazaki I, et al. Immediate transposition of a latissimus dorsi muscle for correcting a postquadrantectomy breast deformity in Japanese patients. Int Surg 1990;75:166-70. [PubMed]
- Kijima Y, Yoshinaka H, Hirata M, et al. Oncoplastic breast surgery combining partial mastectomy with immediate breast reshaping using a keyhole-shaped skin glandular flap for Paget’s disease. Surg Today 2013. [Epub ahead of print]. [PubMed]
- Kijima Y, Yoshinaka H, Hirata M, et al. Oncoplastic surgery for Japanese patients with centrally located breast cancer: partial resection and reconstruction using a local skin-glandular flap. J US-China Med Sci 2011;8:133-7.
- Grisotti A, Calabrese C. Conservation treatment of breast cancer: Reconstructive problems. In: Spear SL. eds. Surgery of the Breast: Principles and Art, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2006:147-78.
- Kijima Y, Yoshinaka H, Hirata M, et al. Oncoplastic surgery combining partial mastectomy with breast reconstruction using a free nipple-areola graft for ductal carcinoma in situ in a ptotic breast: report of a case. Surg Today 2011;41:390-5. [PubMed]
- Spear SL. eds. Breast reduction with the free nipple graft technique. Surgery of the breast, 2nd edition. Philadelphia: Lippincott Williams & Wilkins, 2006:1172-88.
- Oneal RM, Goldstein JA, Rohrich R, et al. Reduction mammoplasty with free-nipple transplantation: indications and technical refinements. Ann Plast Surg 1991;26:117-21. [PubMed]
- Multon O, Bourgeois D, Validire P, et al. Breast cancers with central localization: conservative treatment by tumorectomy with ablation of the areolar plaque. Presse Med 1997;26:988-94. [PubMed]
- Horiguchi J, Koibuchi Y, Iijima K, et al. Local control by breast-conserving surgery with nipple resection. Anticancer Res 2005;25:2957-9. [PubMed]
- Dixon AR, Galea MH, Ellis IO, et al. Paget’s disease of the nipple. Br J Surg 1991;78:722-3. [PubMed]
- Lagios MD, Westdahl PR, Rose MR, et al. Paget’s disease of the nipple. Alternative management in cases without or with minimal extent of underlying breast carcinoma. Cancer 1984;54:545-51. [PubMed]
- Bijker N, Rutgers EJ, Duchateau L, et al. Breast-conserving therapy for Paget disease of the nipple: a prospective European Organization for Research and Treatment of Cancer study of 61 patients. Cancer 2001;91:472-7. [PubMed]
- Marshall JK, Griffith KA, Haffty BG, et al. Conservative management of Paget disease of the breast with radiotherapy: 10- and 15-year results. Cancer 2003;97:2142-9. [PubMed]
- Pezzi CM, Kukora JS, Audet IM, et al. Breast conservation surgery using nipple-areolar resection for central breast cancers. Arch Surg 2004;139:32-7; discussion 38. [PubMed]
- Clough KB, Baruch J. Plastic surgery and conservative treatment of breast cancer. Indications and results. Ann Chir Plast Esthet 1992;37:682-92. [PubMed]