Oncoplastic technique in breast conservative surgery for locally advanced breast cancer
Introduction
Breast-conservation therapy (BCT) is a valuable component of breast cancer surgery in patients who need to preserve the breast and the data show that it has an equivalent survival benefit compared with the conventional mastectomy (1). The lumpectomy and oncoplastic resection are different conceptually. Lumpectomy usually requires a margin of a few millimeters whereas oncoplastic resection usually includes a margin of a few centimeters. Resection of large tumors and locally advanced breast cancer (LABC) can be challenging, in view of the breast conservation surgery (BCS). For making the BCS effective and oncologically safe, there is a need to completely remove all foci of the cancers with an adequate surgical margin width giving enough histological normal tissue and maintaining the cosmetic result of the breast and there are no deformity sequelae.
Inclusion criteria for BCT
The BCT is generally reserved for patients with T1 and T2 tumor. However, the ratio between size of the tumor and the breast is important because the surgeon will plan to remove the tumor with adequate margin and good cosmetic result.
In patients with LABC, giving of neoadjuvant chemotherapy can down stage the tumor for BCS but the surgeon must realize that there are three types of patterns of response after receiving chemotherapy. The first pattern is pathologic complete response in that the gross tumor has totally disappeared. The second pattern is concentric shrinkage in that the tumor has shrunk to a small volume and there is no residual nodule in the peripheral area. The third pattern is mosaic pattern (multifocal residual) in that the tumor has shrunk to small volume like the concentric pattern but it has still many small nodules in the edge of the tumor.
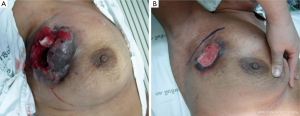
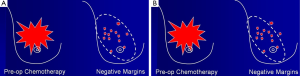
In this condition, BCS is not proper to perform due to high incidence of local recurrence although the tumors will respond well as shown in Figure 1. The total mastectomy is the good procedure for the third pattern of response. When the mastectomy has been done in the mosaic pattern, the margin of resection is crucial because the surgeon can archive the negative margin in two conditions. The first condition is the exact negative margin and there is no residual tumor in the chest wall (Figure 2A). The second one is the presence of foci of tumors outside the skin incision (Figure 2B) but pathological report is also negative margin. The third response can be evaluated by physical examination, mammogram, breast ultrasound and magnetic resonance imaging (MRI). In the patient considering to receive neoadjuvant chemotherapy, photographs and measurement are useful in recording the extent of initial skin lesions such as the small nodules around the primary lesion or area of skin metastases (Figure 3A) because these nodules sometimes disappear after responding to chemotherapy (Figure 3B). Using a radio-opaque marker or tattooing the skin of the breast is another method for identifying the tumor location.
Mammography and ultrasound have been used to evaluate the tumor response after giving neoadjuvant chemotherapy but both techniques cannot differentiate the mass density due to fibrotic lesion of the dead tumor from the viable tumor. The false-positive rates of mammography and breast ultrasound may be 50% or higher (2).
MRI can improve the assessment of neoadjuvant chemotherapy response with sensitivity ranging from 70% to 100% and 50-100% specificity when the tumors respond to chemotherapy, MRI can show the loss of enhancement and MRI is related with pathologic response of residual disease 36-96%. However, MRI cannot detect the absence of residual tumors foci and underestimate the residual noninvasive lesion in the breast following neoadjuvant chemotherapy (3).
The following are selective criteria for selecting candidates for breast-conserving surgery after neoadjuvant chemotherapy (4):
- -Complete resolution of skin edema;
- -Residual tumor size <5 cm;
- -No evidence of multicentric lesion;
- -Absence of extensive intramammary lymphatic invasion/extensive microcalcification.
Cosmetic sequelae after BCS can occur in patients with large tumors and there is a need to remove the large volume of breast tissue. There are three types of cosmetic sequelae after BCS. Type I is asymmetrical breasts with no deformity of the treated breast. Type II is deformity of the treated breast, compatible with partial reconstruction and breast conservation. Type III is major deformity of the breast, requiring mastectomy (5).
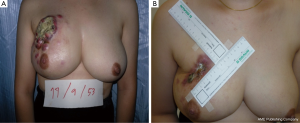
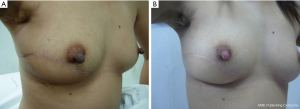
If 20-50% of breast volume resection can be estimated after finishing the operation, cosmetic sequelae type II deformity can occur (Figure 4) (5). Reshaping the breast by using oncoplastic technique such as the latissimus dorsi flap is required to fill the defect after removing large volume of the breast from BCS (6). This oncoplastic technique can prevent and correct the deformity with a good cosmetic outcome (Figure 5).
Absolute contraindication of breast conserving therapy
Absolute contraindications for BCT are as follows (7):
- -Diffuse suspicious or malignant appearing microcalcifications on mammography;
- -Extensive disease that cannot be removed by local excision through a single incision that gets the negative margins with good cosmetic result;
- -Positive pathologic margin;
- -Patients who have received previous radiation to the breast or chest wall;
- -Pregnant women who plan to give the radiation therapy during pregnancy.
The patients who develop breast cancer during pregnancy must avoid radiation therapy due to the internal scatter of the radiation from treatment reaching to the fetus.
Relative contraindication of breast conserving therapy
The following can be considered as relative contraindications of the BCT:
- -Active connective tissue disease especially scleroderma and lupus;
- -Tumor greater than 5 cm in diameter;
- -Focally positive pathologic margins after BCS;
- -Patients ≤35 yr. or patients with a known BRCA1/2 mutation gene.
Patients with systemic lupus erythematosus and scleroderma are significant risk for breast fibrosis with pain and chest wall necrosis.
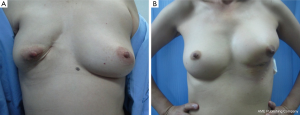
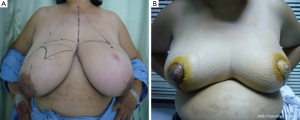
In patients with LABC in which the tumor to breast size ratio is unfavorable is crucial. After removing the tumor in the patients with large breasts, the breast parenchyma defect can be repaired with tissue rearrangement. Reduction mammoplasty techniques can be done at the opposite breast due to symmetry of both sides (Figure 6). This procedure can achieve the greatest benefit from radiation therapy due to reducing the size of the breasts and the patients have a greater degree of dose homogeneity with standard two-dimensional dose compensation techniques.
Margin status in BCS for LABC after neoadjuvant chemotherapy
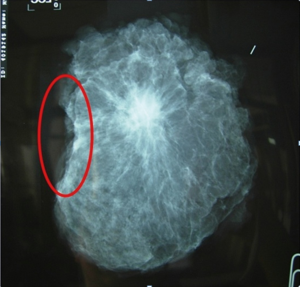
The studies showed BCS for LABC after neoadjuvant chemotherapy is feasible and safe and associated with acceptable local recurrence rates (8-12). As with oncologically breast cancer procedure, the primary goal is to remove the tumor with negative margins. Surgical excision doesn’t attempt to remove the whole previous neoadjuvant volume of lesion because the goal of wide excision is to remove any residual lesion with 1 cm of clear margins. If the lesion after responding to neoadjuvant chemotherapy can be observed in mammography such as microcalcification or spiculated lesion, specimen mammography should be sent to confirm that the whole lesion is removed (Figure 7). If there is no detectable residual lesion in the patient who achieve a clinical complete response, a 2-cm specimen with the metallic marker in the center is suggested (13).
Conclusions
For selected LABC patients (adequate reduction in the tumor size and no evidence of residual nodules in the peripheral area after giving chemotherapy), BCS can be an appropriate local treatment option with acceptable local recurrence rates. Oncoplastic surgery for LABC is safe and effective. Using oncoplastic technique in patients who need to remove the large volume of breast tissue, can prevent and correct the deformity with a good cosmetic outcome.
Acknowledgements
We wish to acknowledge Asst. Prof. Dr. Gloria Vidheecharoen for English revision of the text.
Disclosure: The authors declare no conflict of interest.
References
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [PubMed]
- Sperber F, Weinstein Y, Sarid D, et al. Preoperative clinical, mammographic and sonographic assessment of neoadjuvant chemotherapy response in breast cancer. Isr Med Assoc J 2006;8:342-6. [PubMed]
- Morris EA. Review of breast MRI: indications and limitations. Semin Roentgenol 2001;36:226-37. [PubMed]
- Singletary SE, McNeese MD, Hortobagyi GN. Feasibility of breast-conservation surgery after induction chemotherapy for locally advanced breast carcinoma. Cancer 1992;69:2849-52. [PubMed]
- Clough KB, Cuminet J, Fitoussi A, et al. Cosmetic sequelae after conservative treatment for breast cancer: classification and results of surgical correction. Ann Plast Surg 1998;41:471-81. [PubMed]
- Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375-91. [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [PubMed]
- Jacquillat C, Baillet F, Weil M, et al. Results of a conservative treatment combining induction (neoadjuvant) and consolidation chemotherapy, hormonotherapy, and external and interstitial irradiation in 98 patients with locally advanced breast cancer (IIIA-IIIB). Cancer 1988;61:1977-82. [PubMed]
- Cance WG, Carey LA, Calvo BF, et al. Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg 2002;236:295-302. [PubMed]
- Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001;(30):96-102.
- van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001;19:4224-37. [PubMed]
- Gianni L, Baselga J, Eiermann W, et al. First report of the European Cooperative Trial in operable breast cancer (ECTO): effect of primary systemic therapy. Proc Am Soc Clin Oncol 2002;21:34A(abst 132).
- Hortobagyi GN, Singletary SE, Strom EA. eds. Locally Advanced Breast Cancer. Diseases of the Breast, 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins, 2010:750-1.


