
Primary aldosteronism
Background
It was at the University of Michigan in 1956 that Dr. Jerome Conn first described in a landmark paper a case report describing the physiological manifestations of what he would label primary aldosteronism (PA) (1). The designation of “primary” was meant to distinguish it from secondary forms of aldosterone hypersecretion such as in intra-vascular volume depletion, congestive heart failure, and hepatic cirrhosis. He described a case series of nine patients cured of “potassium-losing nephritis” with the surgical removal of an adrenal cortical adenoma. Indeed, it had only been a few years prior that the hormone aldosterone had first been described and localized to the adrenal cortex (2). In the 1930’s–1940’s, the recognition of a distinct mineralocorticoid produced within the adrenal gland (distinct from cortisone) was established and accepted. Initially named “electrocortin”, the hormone was renamed “aldosterone”, the 18-aldehyde of corticosterone (3,4).
Conn presented a case report of a woman with generalized weakness and transient paralysis, muscle spasms, hypertension, and hypokalemia. It is a case report demonstrating the end stage and progressive effects of the disease process in its most severe form. While it provided a framework for the diagnosis, it perhaps obscured the more common and subtle variants of PA that represent the vast majority of the disease burden. Indeed, until the late 1990’s PA was rarely suspected outside of patients with profound hypokalemia, resulting in much lower estimated prevalence ranges. With the increased adoption of aldosterone to renin ratio as a screening tool, the prevalence estimates increased 5- to 15-fold (5,6).
PA is characterized by excessive aldosterone secretion despite a sodium and volume avid state where renin and angiotensin II are suppressed. The resulting over-activation of the mineralocorticoid receptor has several well studied effects: volume retention, hypertension, hypokalemia, and metabolic alkalosis, as well as a higher risk for developing cardiovascular and kidney disease (3). Sodium is the greatest contributor of serum osmolarity, and total body sodium plays a key role in determining our overall plasma volume (7).
In modern industrialized society, where high sodium diets are commonplace, aldosterone levels are typically low or suppressed. However, studies evaluating hunter-gatherer tribes have demonstrated that in populations with very low dietary sodium intake, aldosterone levels are typically much higher. Chronically elevated aldosterone in these populations is viewed as a normal physiological response to a low sodium (and high potassium) diet, typically does not result in hypertension, and does not result in damage to the cardiovascular or renal systems in the same way that PA does (3). Thus, the unique combination of high aldosterone levels with a volume expanded state in PA ultimately results in a significantly increased risk of death, even when compared to blood pressure-matched control populations (3,8).
At a cellular level, the lipophilic aldosterone traverses the cell membrane and binds the mineralocorticoid receptor (MR) present in the cytoplasm. The aldosterone-MR ligand pair translocates to the nucleus where it is responsible for transcriptional regulation. MR are expressed in the kidney (where their mechanism of action has been well studied), however the receptors are also present in a wide variety of cells including colonic mucosa, eccrine sweat glands, cardiac myocytes, vascular smooth muscle, brown adipose cells, macrophages, and neurons (7). In the kidney, aldosterone promotes the expression of sodium channels in the principal cell, thus induces sodium reabsorption and consequent water reabsorption (7).
Clinical presentation
Hypertension is a common phenomenon in the United States and western populations. Depending upon the diagnostic criterion, an estimated 31.9–45.6% of adults are affected by hypertension with 34.3–36.2% being recommended antihypertensive medication (9). Up to 10% of patients with hypertension have PA whereas this prevalence can exceed 20% in patients with resistant hypertension (10-12). This makes it the most common cause of endocrine hypertension.
Hypertension can be divided into primary (essential) and secondary forms. It is important for clinicians to always consider secondary hypertension, particularly in patients who have resistant hypertension (blood pressure >140/90 mmHg despite being on three antihypertensive agents) (13). Secondary hypertension refers to types of hypertension with an underlying cause from an alternative pathophysiological process, the most common cause of which is PA. Stein and Yee (14) state that there are several situations that should alert the clinician to an underlying diagnosis of PA: new onset hypertension that is resistant or refractory to treatment, new exacerbation of hypertension in a patient with previously mild or controlled hypertension, history of leg cramps, or hypokalemia (spontaneous or diuretic-induced).
It should be stressed that hypokalemia is not a prerequisite for the diagnosis, and in fact is actually uncommon in PA (a fact that was not known until broader screening and implementation of aldosterone to renin ratio testing). Indeed, one major study found only 9–37% of PA patients were hypokalemic (6).
Cardiovascular morbidity associated with PA
Patients with PA experience an increase in cardiovascular morbidity and mortality when compared to patients of similar age or blood pressure (15,16). Cardiovascular effects of PA are varied and severe, including left ventricular hypertrophy, decreases in both systolic and diastolic function, myocardial ischemia, vascular remodeling, arrhythmias, and sudden cardiac death (7). The mechanism of vascular damage extends beyond the hypertension, which in and of itself is a problem for the cardiovascular system. Aldosterone also creates oxidative stress, endothelial inflammation, and vessel damage which produces fibrosis (17).
Renal morbidity associated with PA
PA patients are also at risk for renal disease, frequently manifesting early with tubulointerstitial fibrosis and albuminuria (18). The mechanism of renal decline is thought to be due to renal fibrosis, vascular damage, and injury to the renal podocytes. This results in a decline in glomerular filtration rate (GFR) over time. Chronic kidney disease and end-stage renal disease contribute greatly to the overall morbidity of PA.
Additional morbidity associated with PA
Beyond cardiovascular and renal morbidity, observational studies have demonstrated deleterious effects ranging from metabolic syndrome, skeletal disease, stroke, atrial fibrillation, and cognitive function (3). Associations between Conn Syndrome and diabetes mellitus have been noted since shortly after its discovery in the 1950’s (19,20). Metabolic organ-specific effects of adrenal aldosterone hypersecretion include impaired pancreatic beta cell function, increased hepatic gluconeogenesis, impaired glucose uptake and impaired adipocyte cell differentiation, decreased glucose uptake within skeletal muscle, and increased vascular smooth muscle oxidative stress and endothelial remodeling (19). Further complicating a potential metabolic link is the revelation that some aldosterone producing adenomas often also secrete cortisol (referred to as Connshing Syndrome) (21).
Additional morbidity from PA includes increased probability of obstructive sleep apnea (OSA). While OSA development remains a multifactorial process, the proposed mechanism involves fluid retention from sustained hypertension resulting in peripharyngeal edema and throat mechanical obstruction. A cross-sectional study of PA patients by Wang et al. demonstrated that treatment of PA in this population leads to a reduced probability of OSA symptoms post adrenalectomy (22). As a result, an official guideline recommendation is that all patients with both OSA and hypertension should be screened for PA (13).
PA has also been associated with psychiatric morbidity such as anxiety and depression in numerous small studies in both human and animal models (23). In animal models, mineralocorticoid administration has been demonstrated to induce anxious behavior, with subsequent eplerenone having anxiolytic effect (24,25). Symptoms of mental fatigue, anxiety, and depression detrimentally affect the quality of life of patients with PA (see section: Quality of Life with Primary Aldosteronism, and the effect of treatment) (26).
Epidemiology
The prevalence of PA is controversial and variable in the literature, given the lack of screening in most patients with primary hypertension and spectrum of disease severity (3). Estimates of prevalence within the general population have ranged widely, from 1–20% (27), with increased proportions in the resistant hypertensive population. Factors confounding the determination of prevalence include the possibility of a large amount of subclinical PA in the general population, the heterogeneity in the diagnostic workup, the lack of formalized screening processes, and the propensity for the disease process to have atypical presentations (in fact very few patients present with all the cardinal symptoms of hypertension and hypokalemia) (27). Most cases of PA likely go unrecognized and undiagnosed in the population (28). In a large series from a major academic medical center, less than 3% of patients with resistant hypertension and hypokalemia were screened for PA, suggesting that the screening and diagnosis of PA is abysmal, even at tertiary care centers (29). One large prospective trial of 1,125 newly diagnosed hypertensive patients found an incidence of 4.8% aldosterone producing adenoma and 6.4% idiopathic hyperaldosteronism (for a total of 11.2% of the hypertensive population) (30). The prevalence of PA increases with the severity of hypertension, with one study demonstrating a 3.9% prevalence of PA in stage 1 hypertension and an 11.8% prevalence in stage 3 hypertension (5). A recent review and metanalysis of 36 articles found an incidence range of 3.2–12.7% in primary care settings and 1–29% in tertiary referral centers. Due to substantial study heterogeneity and design flaws, it remains difficult to further narrow the estimated prevalence range (31). While other study estimates have been lower, Douma et al. found in a retrospective observational study that 11.3% of patients with resistant HTN had PA based on salt suppression testing (10), most epidemiological studies have demonstrated a high prevalence and a high degree of under-diagnosis. The true incidence within this high-risk population remains subject to debate due to the challenges of obtaining a proper diagnosis.
Given the degree of under-diagnosis and underappreciated prevalence of PA, the 2016 Endocrine Society’s guidelines called for recognition of PA as a fundamental public health issue. It is estimated that over 99% of PA patients have not received appropriate diagnosis or treatment, and the consequences of lack of treatment are great (including significant cardiovascular and renal risk compared to age and blood pressure matched controls). This has prompted many specialists in the field to strive to improve awareness and access to care (32).
Screening, diagnostic workup and approaches
Given the potential morbidity and mortality of unrecognized hyperaldosteronism, coupled with the widespread availability of effective treatment options, it is imperative for clinicians to recognize and diagnose PA within their at-risk patient populations (3). While confirmatory testing for PA can be complex and often requires referral to either endocrinology specialists or tertiary centers, initial screening for PA is straightforward (28).
Initial workup for suspected PA includes a biochemical evaluation for hyperaldosteronism including testing plasma aldosterone and plasma renin activity. Although variable among experts; a suppressed renin and an aldosterone to renin ratio of greater than 30 is commonly used as the screening diagnostic threshold for PA (33). Alternatively, some laboratories utilize direct renin concentration (as opposed to plasma renin activity). This has several advantages including faster turnaround times, high reproducibility, and can similarly be interpreted as a ratio to plasma aldosterone levels (14). An aldosterone to renin ratio greater than 30 may be also be too high a threshold for confirmation testing, missing many true positives. A practical approach to capture the maximum number of true cases of PA would suggest that any patient with a suppressed renin and a relatively suggestive aldosterone level (i.e., 10 ng/dL or greater) should be considered as a positive screen (3,7).
Serum aldosterone to renin ratio is not without flaws as an initial screening test, with numerous situations creating false positives (hyperkalemia, excessively low renin laboratory thresholds, presence of direct renin inhibitors, use of oral contraceptives) and conversely situations creating false negative results (hypokalemia, mineralocorticoid receptor antagonist use, concurrent use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, diuretic use, sodium restriction, pregnancy) (7). Clinicians should be aware of these drawbacks when interpreting the results of this screening test.
The Endocrine Society guidelines put forth in 2016 are widely considered the gold standard for the management of PA and lay out criteria for screening in at-risk populations. Similarly, the American College of Cardiology and American Heart Association put forth similar hypertension management guidelines in 2017 recommending screening for PA. The following high-risk populations should be screened: patients with resistant hypertension, hypertension with (spontaneous of diuretic-induced) hypokalemia, hypertension with a concurrent adrenal nodule or mass, or hypertension with a family history of early onset hypertension or cerebrovascular accident (13). Many others advocate for a less complex guideline for initial screening, countering that all hypertensives should be screened for PA given the high prevalence of PA in even stage 1 hypertension (34). Screening of all patients with resistant hypertension with computed tomography (CT) abdomen, followed by AVS, has been demonstrated to be a cost effective intervention even when compared to empiric MRA treatment and other medical treatment modalities (35).
Adrenal incidentalomas and PA screening
Incidentally identified adrenal nodules or tumors are frequent on cross sectional imaging, presenting in 0.5–10% of patients undergoing CT scan (with variations in incidence depending on age of the patient) (36,37). While the majority of adrenal incidentalomas are non-functioning benign adenomas, it is important for the clinician to screen for a functioning tumor (such as an aldosteronoma) upon identification of such a lesion. The Endocrine Society recommends that all patients with an incidentally identified adrenal mass and concurrent hypertension be screened for PA (38).
Biochemical workup for PA and confirmatory testing
Patients who screen positive for PA are typically referred to an endocrine or hypertension specialist who will perform confirmatory testing. The caveat is that in patients with a markedly elevated plasma aldosterone, suppressed renin, and hypertension and hypokalemia, the diagnosis of PA is confirmed, and dynamic confirmatory testing is unnecessary (39).
Dynamic confirmatory testing can help avoid costly imaging or unnecessary invasive procedures in the estimated 30–50% of patients with a falsely positive screening examination (39). An oral sodium loading test can frequently be used to confirm the diagnose, as this should suppress aldosterone levels (except in patients with PA) (7). This is commonly performed via oral administration of high doses of salt (6 g per day, for 3–5 days) to achieve a 24-hour urinary sodium excretion >200 mmol. Another commonly utilized option is a saline infusion test (serum aldosterone level is collected after an IV saline infusion of 2 liters over four hours) (40). Additional confirmatory tests include the captopril challenge test (performed by acutely inhibiting the angiotensin converting enzyme) and the fludrocortisone suppression test (sodium loading by a standardized high dose mineralocorticoid administration), which are not commonly utilized in the United States. In all the confirmatory tests, the endpoint of non-suppression of plasma aldosterone indicates a positive test (39). The thresholds used to consider a confirmatory test “positive” vs. “negative” are relatively arbitrary, and are often set to detect the most severe cases of PA, thus representing another challenge for increasing the diagnosis of PA.
Confirmatory testing often requires referral to a specialist or endocrinologist and can require temporary suspending mineralocorticoid antagonists. Discontinuing these medications, even temporarily, in this population can have significant adverse side effects which remains a significant limitation to the classical diagnostic approach (41). An alternative, when confirmatory testing cannot be performed, is to empirically treat with mineralcorticoid receptor antagonists which are indicated for resistant hypertension and PA.
Imaging workup for PA
Once a diagnosis of PA is established, it is important to differentiate which subtype of PA the patient has (42). This can dictate the ultimate therapeutic management. It is necessary to delineate whether it is due a unilateral or bilateral cause, as this will dictate preferred treatment methods. Imaging findings alone (even when CT or MRI demonstrates a unilateral adenoma) is not sufficient for diagnosis, as bilateral adrenal hyperplasia is common in Conn’s syndrome/PA (43). Unilateral sources of PA include adrenal aldosterone producing adenoma, and much less commonly, carcinoma. Carcinoma and adenoma are usually readily differentiated based on size alone, with aldosteronomas rarely being greater than 2 cm in size (and most carcinomas being greater than 6 cm) (Figure 1) (7). Bilateral adrenal hyperplasia effects both adrenal glands typically without a distinct nodule or lesion.
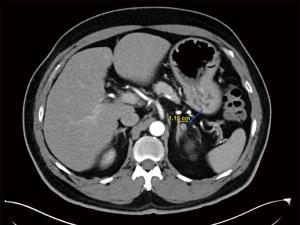
Cross-sectional CT imaging is an essential component of the PA workup. CT provides reliable anatomical information regarding the bilateral adrenal glands, is relatively inexpensive, can exclude an adrenal cortical carcinoma, and is widely accessible (42). In the approximate 40% of PA patients with a hypersecreting adenoma, CT is fairly sensitive in detecting the adenoma. However, CT is not sufficient to confirm a diagnosis of aldosterone-secreting adenoma as it gives no information regarding the physiological or functional status of the tumors (42). Volumetric analysis to differentiate bilateral adrenal hyperplasia versus unilateral adenoma has not been predictive (44). Therefore, while CT is not considered adequate alone, it is an indispensable component of the PA workup to necessarily rule out adrenocortical carcinoma (42). In rare instances of young patients (<35 years old) with a unilateral adenoma and unequivocal presentation, CT may be adequate for subtype differentiation, however in the majority of cases further testing (namely, adrenal venous sampling) is indicated.
Adrenal vein sampling (AVS) is the gold standard to differentiate between unilateral and bilateral forms of PA. When AVS is systematically performed for a diagnosis of PA, unilateral adenomas or hyperplasia amount to 27% of this population and bilateral causes of PA 65–73% (35,45). Despite AVS remaining the “gold standard” for PA subtyping, there remains great interest in the field in finding alternatives which may expand access of care. AVS is invasive, technically demanding, and requires referral to specialized centers. Right adrenal vein cannulation can be difficult, and AVS success rates are estimated to range from 26–81% (46,47). This is believed to create an “eye of the needle” phenomenon which may limit the ability of providers to continue the diagnostic workup (48). The importance of access to AVS for proper disease subtyping is underscored by the fact that in centers without ready access to AVS, a significantly lower percentage of patients are correctly subtyped as having unilateral PA that may be surgically cured (6).
Additional imaging options include functional imaging utilizing radiolabeled PET scans. Original agents such as NP-59 were abandoned due to cost, radiation exposure, and lack of efficacy, however an ongoing push for more effective imaging has produced new effective biomarkers such as 11C-Metomidate. This marker could bind with high avidity and specificity to the enzymes in the adrenal cortex involved in steroid synthesis (CYP11B1, CYP11B2) (42,49). While initial studies have been promising, the broad applicability of 11C-Metomidate is limited by the short half-life of the 11C molecule, requiring an onsite cyclotron (which is cost prohibitive for many centers). New markers such as CDP2230 under investigation promise better sensitivity, specificity, and broader clinical applicability (42).
Pathophysiology and molecular genetics
New insights into the molecular and genetic basis of PA have helped further our understanding of the pathophysiology (14). Aldosterone is produced, under normal conditions, exclusively within the zona glomerulosa, the outermost layer of the adrenal cortex. Aldosterone is produced via oxidation and hydroxylation of deoxycorticosterone by aldosterone synthase (CYP11B2) (48).
Both somatic and germline mutations have been found to be associated with aldosterone producing adenomas (APAs). Somatic mutations (noted in 88% of cases of APAs) include KCNJ5, CACNA1D, ATP1A1, ATP2B3, CACHA1H, and CLCN2 (50-53). These mutations are thought to contribute by activating intracellular signaling pathways that regulate aldosterone production and calcium concentrations. These are the same genes that are thought to be implicated in familial hyperaldosteronism, when found as germline alterations (7).
Immunohistochemistry of resected adrenal glands in PA (typically for aldosterone synthase of CYP11B2) have given us new insights into the localization and pathophysiology of aldosterone excess (48). Whereas subtyping used to be limited to hyperplasia vs. adenoma, we now are able to better understand patterns of aldosterone dysregulation. Aldosterone producing cell clusters (sometimes referred to as APCC’s) represent tiny foci or “islands” of intense aldosterone production, as visible by immunohistochemistry. It has been postulated that APCC’s may act as the precursor lesion to APA (48). Diffusely increased CYP11B2 activity within the adrenal cortex subcapsular region or zona glomerulosa has also been observed, consistent with our classical understanding of hyperplasia. While detailed genetic analysis has increased our understanding for a molecular framework for the disease process, identification of specific somatic mutations does currently guide treatment approach (14).
Treatment approaches
Surgical management
Treatment for PA when it is attributable to a functioning unilateral adrenal adenoma (or unilateral hyperplasia) is predominantly surgical, with the recommended surgical approach being minimally invasive unilateral adrenalectomy. Surgical treatment offers good result with a relatively low complication profile. The laparoscopic adrenalectomy offers numerous advantages over traditional open surgery including less pain, faster return of bowel function, and shorter hospital length of stay. For this reason, minimally invasive adrenalectomy is the preferred approach for benign tumors such as aldosterone-producing adenomas (APAs) (Figure 2) (54,55). Studies comparing medical management against surgery for patients with unilateral adenoma have found that surgery results in a significantly higher complete control of hypertension (87.5% vs. 13.3%), improved cardiovascular outcomes at 2.5 years (56), and improved renal outcomes (18), suggesting that in this subtype population surgery should remain first line of treatment (57,58). Patients who are not deemed surgical candidates are often treated medically in the same manner of those patients with bilateral PA (i.e., with MRAs).

While it is generally considered to have a steeper learning curve than traditional transperitoneal approach, a posterior retroperitoneoscopic adrenalectomy (PRA) has become increasingly popular as a minimally invasive approach to these tumors. This approach has the benefit of avoiding the peritoneal cavity, particularly in patients with prior abdominal scar tissue or surgeries. This approach has been found to be safe and equally effective to transperitoneal approach and may have additional benefits such as earlier resumption of diet and activity (59,60).
Alternative non-surgical approaches such as percutaneous or image-guided ablation techniques may offer similar levels of efficacy for adrenal adenomas in patients that cannot tolerate general anesthesia (61). One disadvantage of this approach is the unavailability of histopathology and diagnosis confirmation post-procedure.
There are several perioperative considerations for surgeons to be aware of when performing adrenalectomy for APA. Postoperative adrenal insufficiency and hyperkalemia should be screened for, particularly in patients with contralateral suppression on AVS (62,63). MRAs should be discontinued in the postoperative setting and both blood pressure and electrolytes should be monitored closely.
Medical treatment options for hyperaldosteronism
Medical treatment is typically with mineralocorticoid receptor antagonists (MRAs) such as spironolactone or eplerenone. MRAs are recommended as first-line treatment when tolerated in non-lateralized forms of PA, and in patients with lateralized forms that decline surgery (or are not deemed appropriate surgical candidates) (64). Spironolactone has numerous side effects independent of the mineralocorticoid system, most prominently affecting the sex hormones. It is an antagonist of the androgen and progesterone receptors, and enhances testosterone aromatization into estradiol, increases testosterone clearance. Side effects, such as gynecomastia in males and menstrual dysfunction in females can occur (64). For those who cannot tolerate spironolactone, alternative agents such as eplerenone can be utilized (64). Additional second line agents include amiloride and dihydropyridinic calcium channel blockers, some of which are MR antagonists (64).
While most studies demonstrate risk reduction with the implementation of an appropriate medical regimen for PA, outcomes in patients treated with MRA therapy remain inferior to hypertensive controls. Patients on MRA for PA, when compared to hypertensive controls, continue to have a nearly two-fold increased risk of adverse cardiovascular event, significantly higher risk of death, and higher rates of atrial fibrillation and diabetes mellitus (58). On subgroup analysis, it was demonstrated that ongoing suppression of plasma renin activity while on MRA is a risk factor for deleterious outcome in PA (58). Thus, an alternative interpretation is that MRA therapy can be effective at lowering incident cardiovascular risk, but one potential determinant of this efficacy is a rise in renin from a suppressed to unsuppressed level.
Defining successful treatment and remission of PA
Defining successful cure after medical or surgical treatment of PA has been challenging as well due to heterogeneity in the proposed criteria for success. In 2016 the Primary Aldosteronism Surgical Outcomes (PASO) group convened as an international multidisciplinary panel to establish consensus regarding these criteria (65). In broad terms, cure can be defined using clinical or biochemical parameters and can be subdivided into “complete”, “partial”, or “missing”. The most stringent definitions for cure using the PASO calls for normokalemia, normalization of aldosterone to renin ratio (ARR), normal blood pressure, and cessation of all blood pressure medications (66). Partial success can be obtained with a reduction in blood pressure medications, improved blood pressure on the same medication regimen, and/or abnormal but improved plasma aldosterone levels. The PASO group reported complete clinical success in 37% undergoing adrenalectomy, partial clinical success in an additional 47%, and complete biochemical success in 94%. Younger patients, female patients, and patients on less preoperative blood pressure medications had a higher likelihood of postoperative complete clinical success (65).
Quality of life with PA, and the effect of treatment
Beyond objective outcome measures, new research has aimed to determine the impact of PA on patient-centered outcomes and quality of life. Increasingly recognized as an important health status marker, health-related quality of life (QOL) attempts to view the disease process through a broader psychosocial lens to determine its complete impact on affected patients. As previously mentioned, PA has been linked to psychiatric co-morbidities including depression and anxiety (23,26). One prospective study found significantly higher QOL scores amongst PA patients undergoing treatment at 3 and 6 months, with highest QOL scores going to those undergoing adrenalectomy (67). This is concurrent with other studies that have found QOL benefit with treatment regardless, but an increased and more immediate benefit in the subset undergoing laparoscopic adrenalectomy compared to MRA therapy alone (68,69).
Special considerations including pregnancy and atrial fibrillation
Hypertension is very common during pregnancy, however the incidence of PA within the pregnant population is uncertain. Normal physiological changes during pregnancy to the renin angiotensin system can make the diagnosis during pregnancy exceptionally difficult. PA during pregnancy is associated with numerous maternal and fetal complications and is a high risk for pregnancy. Further complicating management, spironolactone is considered teratogenic and is typically held during pregnancy. Adrenalectomy is rarely indicated in pregnancy for cases of refractory hypertension, however can be performed safely during pregnancy when indicated (70,71).
Recent evidence has demonstrated that aldosterone plays an important in the pathogenesis of atrial fibrillation via cardiac modeling (left atrium dilation, diastolic dysfunction), hypertrophy, inflammation, and fibrosis. It should therefore come as no surprise that patients with PA have a 7–12-fold higher risk of atrial fibrillation than control patients with essential hypertension. The development of atrial fibrillation plays an important role in explaining the worse cerebrovascular and cardiovascular outcomes in the PA population (35). Moreover, appropriate treatment of PA with surgical adrenalectomy or appropriate MRA therapy may mitigate this increased risk of atrial fibrillation (72).
Conclusions
Our understanding of PA has rapidly grown since it was first recognized little over 60 years ago. However, PA continues to be vastly under-recognized and under-diagnosed despite effective medical and surgical treatment options being available. Our understanding of the disease process has evolved to include increased knowledge of the genetic factors that drive the disease, as well as improvements in screening, imaging, and treatment modalities. The cardiovascular, renal, and cerebrovascular consequences of delayed diagnosis and prolonged untreated PA are too great to be ignored. Patients will greatly benefit from clinicians who recognize the many patients who might be suffering from undiagnosed hyperaldosteronism and can pursue an appropriate diagnostic workup when applicable. While it remains one of the few forms of hypertension with an available, cost-effective, cure, few reach the point of treatment and there remains much work to be done to improve outcomes in this population. Clinicians should continue to increase screening for PA in at-risk populations, increase referrals to available specialists for any positive or equivocal screens, and when in doubt, consider empiric treatment with MRAs.
Acknowledgments
The authors would like to thank the editors of Gland Surgery for the opportunity, and to Lia Wrenn MD, for her support and critical appraisal of the manuscript. We would like to thank Dr. Edward Borrazzo and Dr. Matthew Nehs for contributing images to the manuscript.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Conn JW, Louis LH. Primary aldosteronism, a new clinical entity. Ann Intern Med 1956;44:1-15. [Crossref] [PubMed]
- Simpson SA, Tait JF, Bush IE. Secretion of a salt-retaining hormone by the mammalian adrenal cortex. Lancet 1952;2:226. [Crossref] [PubMed]
- Vaidya A, Mulatero P, Baudrand R, et al. The Expanding Spectrum of Primary Aldosteronism: Implications of Diagnosis, Pathogenesis, and Treatment. Endocr Rev 2018;39:1057-88. [Crossref] [PubMed]
- Kuizenga MH, Cartland GF. Fractionation studies on adrenal cortical extract with notes on the distribution of biological activity among the crystalline and amorphous fractions. Endocrinology 1939;24:526-35. [Crossref]
- Monticone S, Burrello J, Tizzani D, et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol 2017;69:1811-20. [Crossref] [PubMed]
- Mulatero P, Stowasser M, Loh KC, et al. Increased Diagnosis of Primary Aldosteronism, Including Surgically Correctable Forms, in Centers from Five Continents. J Clin Endocrinol Metab 2004;89:1045-50. [Crossref] [PubMed]
- Byrd JB, Turcu AF, Auchus RJ. Primary Aldosteronism: Practical Approach to Diagnosis and Management. Circulation 2018;138:823-35. [Crossref] [PubMed]
- Reincke M, Fischer E, Gerum S, et al. Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension 2012;60:618-24. [Crossref] [PubMed]
- Muntner P, Carey RM, Gidding S, et al. Potential U.S. Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. J Am Coll Cardiol 2018;71:109-18. [Crossref] [PubMed]
- Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet 2008;371:1921-6. [Crossref] [PubMed]
- Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomized, double-blind, crossover trial. Lancet 2015;386:2059-68. [Crossref] [PubMed]
- Calhoun DA, Nishizaka MK, Zaman MA, et al. Hyperaldosteronism among black and white patients with resistant hypertension. Hypertension 2002;40:892-6. [Crossref] [PubMed]
- Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:1269-324. [Crossref] [PubMed]
- Stein DL, Yee J. Dr. Conn Lives on: Insights Into Screening and Genetics of Primary Aldosteronism. Adv Chronic Kidney Dis 2019;26:81-4. [Crossref] [PubMed]
- Monticone S, D’Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2018;6:41-50. [Crossref] [PubMed]
- Hill MA, Sowers JR. Mineralocorticoid antagonists and ENaC inhibitors in hyperaldosteronism. J Clin Hypertens (Greenwich) 2019;21:929-31. [Crossref] [PubMed]
- Chen ZW, Hung CS, Wu VC. Primary Aldosteronism and Cerebrovascular Diseases. Endocrinol Metab (Seoul) 2018;33:429-34. [Crossref] [PubMed]
- Hundemer GL, Curhan GC, Yozamp N, et al. Renal Outcomes In Medically and Surgically Treated Primary Aldosteronism. Hypertension 2018;72:658-66. [Crossref] [PubMed]
- Bothou C, Beuschlein F, Spyroglou A. Links between aldosterone excess and metabolic complications: A comprehensive review. Diabetes metab. 2019. [Crossref] [PubMed]
- Conn JW, Knopf RF, Nesbit RM. Clinical Characteristics of Primary Aldosteronism from an analysis of 145 cases. Am J Surg 1964;107:159-72. [Crossref] [PubMed]
- Beuschlein F, Reincke M, Arlt W. The impact of Connshing’s syndrome- mild cortisol excess in primary aldosteronism drives diabetes risk. J Hypertens 2017;35:2548. [Crossref] [PubMed]
- Wang E, Chomsky-Higgins K, Chen Y, et al. Treatment of Primary Aldosteronism Reduces the Probability of Obstructive Sleep Apnea. J Surg Res 2019;236:37-43. [Crossref] [PubMed]
- Sonino N, Fallo F, Fava GA. Psychological aspects of primary aldosteronism. Psychother Psychosom 2006;75:327-30. [Crossref] [PubMed]
- Hlavacova N, Bakos J, Jezova D. Eplerenone, a selective mineralcorticoid receptor blocker, exerts, anxiolytic effects accompanies by changes in the stress hormone release. J Psychopharmacol 2010;24:779-86. [Crossref] [PubMed]
- Hlavacova N, Jezova D. Chronic treatment with the mineralocorticoid hormone aldosterone results in increased anxiety-like behavior. Horm Behav 2008;54:90-7. [Crossref] [PubMed]
- Künzel HE, Apostolopoulou K, Pallauf A, et al. Quality of life in patients with primary aldosteronism: Gender differences in untreated and long-term treated patients and associations with treatment and aldosterone. J Psychiatr Res 2012;46:1650-4. [Crossref] [PubMed]
- Adlin EV. Subclinical Primary Aldosteronism. Ann Intern Med 2017;167:673-4. [Crossref] [PubMed]
- Funder JW. Mineralocorticoid receptor antagonists: emerging roles in cardiovascular medicine. Integr Blood Press Control 2013;6:129-38. [Crossref] [PubMed]
- Ruhle BC, White MG, Alsafran S, et al. Keeping primary aldosteronism in mind: Deficiencies in screening at-risk hypertensives. Surgery 2019;165:221-7. [Crossref] [PubMed]
- Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 2006;48:2293-300. [Crossref] [PubMed]
- Käyser SC, Dekkers T, Groenewoud HJ. Study Heterogeneity and Estimation of Prevalance of Primary Aldosteronism: A Systematic Review and Meta-Regression Analysis. J Clin Endocrinol Metab 2016;101:2826-35. [Crossref] [PubMed]
- Funder JW. Reply to the authors: Primary aldosteronism as a public health issue. Lancet 2016;4:972-3. [PubMed]
- Harris DA, Au-Yong I, Basnyat PS, et al. Review of surgical management of aldosterone secreting tumours of the adrenal cortex. Eur J Surg Oncol 2003;29:467-74. [Crossref] [PubMed]
- Maiolino G, Calo LA, Rossi GP. The Time has Come for Systematic Screening for Primary Aldosteronism in All Hypertensives. J Am Coll Cardiol 2017;69:1821-3. [Crossref] [PubMed]
- Lubitz CC, Economopoulos KP, Sy S, et al. Cost-Effectiveness of Screening for Primary Aldosteronism and Subtype Diagnosis in the Resistant Hypertensive Patients. Circ Cardiovasc Qual Outcomes 2015;8:621-30. [Crossref] [PubMed]
- Buffolo F, Monticone S, Burrello J. Is Primary Aldosteronism Still Largely Unrecognized? Horm Metab Res 2017;49:908-14. [Crossref] [PubMed]
- Vaidya A, Hamrahian A, Bancos I, et al. The Evaluation of Incidentally Discovered Adrenal Masses. Endocr Pract 2019;25:178-92. [Crossref] [PubMed]
- Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 2016;101:1889-916. [Crossref] [PubMed]
- Reznik Y, Amar L, Tabarin A. SFE/SFHTA/AFCE consensus of primary aldosteronism, part 3: Confirmatory testing. Ann Endocrinol (Paris) 2016;77:202-7. [Crossref] [PubMed]
- Rossi GP, Belfiore A, Bernini G, et al. Prospective evaluation of the saline infusion test for excluding primary aldosteronism due to aldosterone-producing adenoma. J Hypertens 2007;25:1433-42. [Crossref] [PubMed]
- Fischer E, Beuschlein F, Bidlingmaier M, et al. Commentary on the Endocrine Society Practice Guidelines: Consequences of adjustment of antihypertensive medication in screening for primary aldosteronism. Rev Endocr Metab Disord 2011;12:43-8. [Crossref] [PubMed]
- Lenders JWM, Eisenhofer G, Reincke M. Subtyping of Patients with Primary Aldosteronism: An Update. Horm Metab Res 2017;49:922-8. [Crossref] [PubMed]
- Wachtel H, Bhandari S, Roses R, et al. Primary aldosteronism with nonlocalizing imaging. Surgery 2019;165:211-8. [Crossref] [PubMed]
- Degenhart C, Schneller J, Osswald A, et al. Volumetric and densitometric evaluation of the adrenal glands in patients with primary Aldosteronism. Clin Endocrinol (Oxf) 2017;86:325-31. [Crossref] [PubMed]
- Westerdahl C, Bergenfelz A, Isaksson A, et al. Primary Aldosteronism among newly diagnosed and untreated hypertensive patients in a Swedish primary care area. Scand J Prim Health Care 2011;29:57-62. [PubMed]
- Fujii Y, Umakoshi H, Wada N, et al. Subtype prediction of primary aldosteronism by combining aldosterone concentrations in the left adrenal vein and inferior vena cava: a multicenter collaborative study on adrenal venous sampling. J Hum Hypertens 2017;32:12-9. [Crossref] [PubMed]
- Rossi GP, Barisa M, Allolio B, et al. The Adrenal Vein Sampling International Study (AVSIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab 2012;97:1606-14. [Crossref] [PubMed]
- Holler F, Heinrich DA, Adolf C, et al. Steroid Profiling and Immunohistochemistry for Subtyping and Outcome Prediction in Primary Aldosteronism: A Review. Curr Hypertens Rep 2019;21:77. [Crossref] [PubMed]
- Hennings J, Lindhe O, Bergstrom M, et al. [11C]metomidate positron emission tomography of adrenocortical tumors in correlation with histopathological findings. J Clin Endocrinol Metab 2006;91:1410-4. [Crossref] [PubMed]
- Nanba K, Omata K, Gomez-Sanchez CE, et al. Genetic Characteristics of Aldosterone-Producing Adenomas in Blacks. Hypertension 2019;73:885-92. [Crossref] [PubMed]
- Beuschlein F, Boulkroun S, Osswald A, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet 2013;45:440-4. [Crossref] [PubMed]
- Azizan EA, Poulsen H, Tuluc P, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet 2013;45:1055-60. [Crossref] [PubMed]
- Nanba K, Omata K, Else T, et al. Targeted Molecular Characterization of Aldosterone-Producing Adenomas in White Americans. J Clin Endocrinol Metab 2018;103:3869-76. [Crossref] [PubMed]
- Brunt LM, Doherty GM, Norton JA, et al. Laparoscopic adrenalectomy compared to open adrenalectomy for benign adrenal neoplasms. J Am Coll Surg 1996;183:1-10. [PubMed]
- Hazzan D, Shiloni E, Golijanin D, et al. Laparoscopic vs open adrenalectomy for benign adrenal neoplasm. Surg Endosc 2001;15:1356-8. [Crossref] [PubMed]
- Bernini G, Bacca A, Carli V, et al. Cardiovascular changes in patients with primary aldosteronism after surgical or medical treatment. J Endocrinol Invest 2012;35:274-80. [PubMed]
- Meng X, Ma WJ, Jiang XJ, et al. Long-term blood pressure outcomes of patients with adrenal venous sampling-proven unilateral primary aldosteronism. J Hum Hypertens 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Hundemer GL, Curhan GC, Yozamp N, et al. Cardiometabolic Outcomes and Mortality in Medically Treated Primary Aldosteronism: A Retrospective Cohort Study. Lancet Diabetes Endocrinol 2018;6:51-9. [Crossref] [PubMed]
- Arezzo A, Bullano A, Cochetti G, et al. Transperitoneal versus retroperitoneoscopic adrenalectomy for adrenal tumours in adults. Cochrane Database Syst Rev 2018;12:CD011668. [PubMed]
- Nigri G, Rosman AS, Petrucciani N, et al. Meta-analysis of trials comparing laparoscopic transperitoneal and retroperitoneal adrenalectomy. Surgery 2013;153:111-9. [Crossref] [PubMed]
- Liu SY, Chu CC, Tsui TK, et al. Aldosterone-producing Adenoma in Primary Aldosteronism: CT-guided Radiofrequency Ablation- Long-term Results and Recurrence Rate. Radiology 2016;281:625-34. [Crossref] [PubMed]
- Tahir A, McLaughlin K, Kline G. Severe hyperkalemia following adrenalectomy for aldosteronoma: prediction, pathogenesis and approach to clinical management- a case series. BMC Endocrine Disorders 2016;16:43. [Crossref] [PubMed]
- Shariq OA, Bancos I, Cronin PA, et al. Contralateral suppression of aldosterone at adrenal venous sampling predicts hyperkalemia following adrenalectomy for primary aldosteronism. Surgery 2018;163:183-90. [Crossref] [PubMed]
- Pechère-Bertschi A, Herpin D, Lefebvre H. SFE/SFHTA/AFCE consensus on primary Aldosteronism, part 7: Medical treatment of primary Aldosteronism. Annales d’Endocrinologie 2016;77:226-34. [Crossref] [PubMed]
- Williams TA, Lenders JWM, Mulatero P, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet 2017;5:689-99. [PubMed]
- Miller BS, Turcu AF, Nanba AT, et al. Refining the Definitions of Biochemical and Clinical Cure for Primary Aldosteronism Using the Primary Aldosteronism Surgical Outcome (PASO) Classification System. World J Surg 2018;42:453-63. [Crossref] [PubMed]
- Ahmed AH, Gordon RD, Sukor N, et al. Quality of Life in Patients with Bilateral Priamry Aldosteronism before and during Treatment with Spironolactone and/or Amiloride, Including a Comparison with Our Previously Published Results in Those with Unilateral Disease Treated Surgically. J Clin Endocrinol Metab 2011;96:2904-11. [Crossref] [PubMed]
- Velema M, Dekkers T, Hermus A, et al. Quality of Life in Primary Aldosteronism: a Comparative Effectiveness Study of Adrenalectomy and Medical Treatment. J Clin Endocrinol Metab 2018;103:16-24. [PubMed]
- Sukor N, Kogovsek C, Gordon RD, et al. Improved Quality of Life, Blood Pressure, and Biochemical Status Following Laparoscopic Adrenalectomy for Unilateral Primary Aldosteronism. J Clin Endocrinol Metab 2010;95:1360-4. [Crossref] [PubMed]
- Landau E, Amar L. Primary Aldosteronism and pregnancy. Annales d’Endocrinologie 2016;77:148-60. [Crossref] [PubMed]
- Shiraishi K, Kikuta K, Nitta Y, et al. Laparoscopic adrenalectomy due to primary aldosteronism during pregnancy. Hinyokika Kiyo 2014;60:381-5. [PubMed]
- Hundemer GL, Curhan GC, Yozamp N, et al. Incidence of Atrial Fibrillation and Mineralocorticoid Receptor Activity in Patients With Medically and Surgically Treated Primary Aldosteronism. JAMA Cardiol 2018;3:768-74. [Crossref] [PubMed]


