Sensory nerves and pancreatitis
Introduction
Pancreatitis, which can be generally classified into two forms: acute pancreatitis (AP) and chronic pancreatitis (CP), is an inflammatory reaction of the pancreatic tissue. Either form of pancreatitis is serious and can lead to complications. In severe cases, bleeding, infection, and permanent tissue damage may occur.
The function of primary sensory neurons is to receive information from external environment and transmit it to the central nervous system (CNS), and activation of these neurons causes the release of neurotransmitters from peripheral endings; this process is a basis for the local “axon reflex” (1). Sensory nerves have a special sensitivity to capsaicin (2). Capsaicin, a natural product of the hot pepper plant, is structurally similar to endogenous lipid molecules such as arachidonic acid, specifically activating the transient receptor potential vanilloid 1 (TRPV1) on primary sensory nerves (3,4). TRPV1 is a nonselective-cation channel with a preference for calcium that is mainly expressed on primary spinal afferent neurons containing the neuropeptides calcitonin gene-related peptide (CGRP), substance P (SP) (5). Low doses of capsaicin through binding to the TRPV1 result in the activation of sensory nerves accompanied by the release of neuromediators such as CGRP, tachykinins (6,7), whereas high neurotoxic doses of capsaicin lead to ablation of sensory nerves with the decrease in plasma and tissue level of CGRP (8) and the disturbances of pancreatic microcirculation (9). Sensory nerves are strongly implicated in the maintenance of pancreatic integrity. Deactivation of sensory nerves aggravated AP, leading to the hypoxia and pancreatic damage, whereas stimulation of these nerves increased pancreatic resistance against acute inflammation (10-12). The purpose of this review is to summarize the relationship of sensory nerves and pancreatitis.
Innervation of the pancreas
The innervation of the digestive tract is composed of intrinsic component known as the enteric nervous system (ENS) and extrinsic component. The extrinsic nerve fibers can be anatomically and functionally classified in afferent nerves, conducting sensory information from the digestive system to the CNS, and efferent nerves, through which the CNS convey motor command to the digestive system (13).
The pancreas is richly innervated by a variety of myelinated or unmyelinated nerve fibers, thick nerve bundles and aggregates of neural cell bodies known as intrapancreatic ganglia; these ganglionic structures representing the intrinsic component of the pancreatic innervation are randomly distributed throughout the pancreatic parenchyma (13). The afferent system, which involved in transmitting sensory to the CNS, is composed of thin unmyelinated fibers. These fibers run with either the parasympathetic pathways (vagi) or the sympathetic inputs (splanchnic nerves), of which cell bodies processes can be located either in the dorsal root ganglia (DRG) (the spinal afferents) or in the nodose ganglia (NG) (vagal afferents) (13-15). The extrinsic fibers belong to the sympathetic and parasympathetic systems (14). Parasympathetic fibers originating mainly from the dorsal vagal nucleus and partly from the ambigous nucleus of the brain stem are carried in the vagus nerve with termination to synapse upon the intrapancreatic ganglia. Postganglionic parasympathetic fibers from these ganglia reach both exocrine and endocrine pancreas after being distributed with sympathetic fibers. Preganglionic sympathetic fibers are conducted through the splanchnic nerves to synapse in the celiac ganglia. Postganglionic sympathetic fibers run with the blood vessels to the pancreas. Many of their terminations are close to the blood vessels controlling pancreatic blood flow (14,15).
The afferent nerves contain a variety of specific receptors and ion channels which are the molecular site of activation (see Figure 1). DRG and NG neurons have been thought to convey different types of sensory information from the viscera to the CNS. DRG neurons respond to physiological levels of stimulation and are capable of encoding noxious input. Therefore, they are believed to mediate painful sensations, and their close association with the vasculature also suggests an efferent/cytoprotective role in response to injury or inflammation (16). NG neurons have low thresholds for activation, and previous studies have suggested that these afferents do not code into the noxious range (17). Therefore it was believed that these afferents could only be involved in the physiological regulation of visceral stimulation, including emotional and behavioral aspects. Data have been accumulating over the past decade showing that persistent inflammatory pain requires the expression of TPRV1 in primary afferents. TRPV1 was expressed in both DRG and NG pancreatic neurons, but at a significantly lower level in the NG (79% for DRG vs. 35% for NG) (18). Ablation of TRPV1-positive fibers via application of resiniferatoxin to celiac ganglia significantly reduced SP release and inflammation in a rat model of pancreatitis (19). TRPV1 mRNA and protein expression were both increased in pancreatic DRG neurons following induction of CP in rats, and treatment with a TRPV1 antagonist reduced both visceral and referred somatic pain behaviors (20). The afferent nerves contain a variety of specific receptors and ion channels which are the molecular site of activation (see Figure 1).
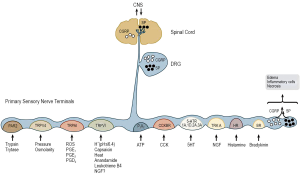
Effects of sensory nerves on pancreatic exocrine secretion
Pancreatic exocrine secretion is regulated by a complex neurohormonal system, and the neural regulation of this secretion including both the CNS, the afferent (sensory) and efferent (motor) vagus and sympathetic nerves and the ENS innervating the stomach, duodenum and pancreas (21-24). Autonomic nerves of the pancreas form a separate “pancreatic brain”, which is a part of the ENS and the center of short enteric reflexes, are essential to regulate pancreatic secretory function and microcirculation (24,25). The activation of dorsal vagal complex (DVC) of the brainstem, which is the core of vago-vagal, cholinergic entero-pancreatic reflex, determines the central regulation of pancreatic exocrine secretion (14,22). DVC integrates signals from olfactory cortex and hypothalamus with inputs originating from the intestine via vagal afferent nerves and transmits outputs to the pancreas to produce secretory response to food ingestion (25,26). Physiologic stimuli entering into the duodenum stimulate the release of intestinal hormones and activate enteric-pancreatic reflex mechanisms to stimulate or inhibit exocrine pancreatic secretion.
Cholecystokinin (CCK), a peptide hormone both in the gastrointestinal tract throughout the human small intestine and nerves in the myenteric plexus of the ENS and in the CNS, has been studied for many years as a major pancreatic secretagogue (27). CCK could stimulate pancreatic secretion by two following possible mechanisms. First, CCK binds CCK-1 receptors on pancreatic acinar cells causing the release of pancreatic enzymes. Second, CCK binds CCK-1 receptors on capsaicin-sensitive vagal afferent fibers. Stimulation of vagal afferent nerves generates a signal sent to the medial nucleus tractus solitarius (NTS) which locates in the brain stem and output information is transmitted through cholinergic postganglionic vagal efferent fibers to the pancreas. Acetylcholine released from the efferent nerve endings, binds M3 muscarinic receptors on the pancreatic acinar cells and causes the release of pancreatic enzymes (28). However, the effect of CCK directly acting on pancreatic acinar cells remains controversial. CCK has been demonstrated to stimulate pancreatic exocrine secretion in mouse, rat and dog through directly binding CCK-1 receptors on acinar cells, whereas human acinar cells have been thought to lack this activity, due to inadequate number of CCK-1 receptors on human acinar cells (29,30). Sensory nerves are involved in neural-reflex stimulation of pancreatic exocrine secretion by CCK and deactivation of these nerves with capsaicin diminishes the pancreatic secretory response to food (24).
Serotonin secreting cells, which releasing serotonin in response to wide range of stimuli, distribute throughout the gastrointestinal tract. It has been shown that 5-HT1P receptor agonists inhibit amylase secretion (31) while 5-HT3 agonists stimulate exocrine stimulation via vagal afferent fibers (32).
Melatonin, of which receptors are present on cells in the exocrine and endocrine pancreas (33), is known to stimulate enzyme release from pancreas depending on CCK release and stimulation of vagal afferents since inhibition of either signal was accompanied by a loss in amylase release.
C-natriuretic peptide (CNP) is a 22 amino acid peptide that is abundant in the CNS and gastrointestinal tract. The CNP receptor is a member of the G protein coupled receptor family locating on both acinar and duct cells of the pancreas (34). It has been shown that CNP increased pancreatic protein, chloride and fluid secretion without affecting bicarbonate secretion. However, the secretion of chloride is decreased in rats following truncal vagotomy or perivagal application of capsaicin, suggesting that this function of CNP is modulated by the parasympathetic nervous system (35).
Application of leptin or ghrelin into the duodenal lumen significantly and dose-dependently increased pancreatic amylase secretion and raised CCK plasma level. Stimulatory effects of these substances on exocrine pancreas through activation of entero-pancreatic reflex via CCK release (36).
Effects of sensory nerves on AP
Effect on the improvement of pancreatic microcirculation
AP is characterized by an inflammatory reaction of the pancreatic tissue and disturbances of pancreatic microcirculation (37). It is well known that microcirculatory disturbances play a pivotal role in the initiation and progression of mild necrotizing pancreatitis to severe necrotizing pancreatitis (SNP) (38). Reduction of blood flow in the pancreatic microcirculation could be the primary reason of pancreatic inflammatory changes, leading to the capillary thrombosis, ischemia, activation of macrophages and leukocytes, release of pro-inflammatory cytokines and the accumulation of oxygen-derived free radicals and toxic substances in the pancreas (11,39-41).
Sensory nerves are responsible for the regulation of pancreatic blood flow and stimulation of sensory nerves could produce the hyperemia in the pancreatic microcirculation (11). As it has been mentioned above that stimulation of pancreatic sensory nerves releases a variety of neurotransmitters such as SP, CGRP; and these neurotransmitters could cause the local vasodilatation as vasodilatatory neuropeptide. The protective effects of sensory nerve stimulation or CGRP administration on pancreas have been attributed, at least in part, to the improvement of pancreatic circulation (10,42). Stimulation of sensory nerves protects the pancreas against damage evoked by ischemia reperfusion (I/R), whereas ablation of sensory nerves aggravates pancreatic tissue damage in the pancreas exposed to I/R. The beneficial effect of sensory nerve stimulation on maintenance of pancreatic integrity is partly dependent on improving the pancreatic blood flow by releasing CGRP (11). CGRP binds to its receptors CGRP1 and CGRP2 inducing a raise of intracellular cAMP, followed by the activation of protein kinase A, opening of K+ channels and subsequent relaxation of smooth muscle cells (43).
However, Warzecha et al. (44,45) demonstrated that CGRP administration aggravated pancreatic injury in edematous pancreatitis after induction of pancreatitis. In acute edematous pancreatitis, hyperemia is a well-known microcirculatory disturbance in the pancreatic microcirculation characterized by capillary leakage, edema formation (38). Therefore, CGRP may aggravate pancreatic damage by augmenting pancreatic blood flow. In contrast, acute necrotizing pancreatitis is characterized by hypoperfusion of the pancreas leading to inflammation and pancreatic damage (38). This can be attenuated by CGRP administration through improving blood flow in pancreatic microcirculation.
Liddle RA (46,47) also has demonstrated that stimulation of primary sensory neurons produces local vasodilation, plasma extravasation, and pain and is due largely to the release of the tachykinins SP and CGRP. SP and CGRP, which appear to be responsible for many of the features of neurogenic inflammation, have the abilities to interact with endothelial cells, arterioles, mast cells and other immune cells to induce vasodilation, edema and inflammatory cell infiltration.
Effect on inflammatory reaction of pancreas
It has been reported that activation of sensory nerves is able to increase the production of insulin-like growth factor 1 (IGF-1) in many tissues, and could reduce cell apoptosis (48). Previous studies on the rats have shown that IGF-1 attenuates pancreatic damage in AP and this protective effect is related to the stimulation of an anti-inflammatory interleukin-10 (IL-10) production. The function of IL-10 is to inhibit the activation of macrophages and T cells and to decrease the pro-inflammatory cytokines production in AP (49). Application of exogenous growth hormone (GH) to the rats with active sensory nerves stimulates IGF-1 release and attenuates acute pancreatic damage as is manifested by significant decrease of pancreatic edema, reduction of lipase and TNF-alpha blood levels, and improvement of pancreatic blood flow; while deactivation of sensory nerves by capsaicin completely reversed above protective influence of GH on AP (50). Capsaicin could significantly induces the liberation of CGRP by activating sensory nerves, leading to the reduction of activation of inflammatory leukocytes and the release of proinflammatory cytokines (51). IL-1 is a well-known proinflammatory cytokine. It plays a pivotal role in the production of systemic acute phase responses and the release of other proinflammatory cytokine cascade (52). Dembiński et al. (11) have shown that the protective effect of sensory nerve on necrotizing pancreatitis is manifested by a decrease in plasma amylase and lipase activity, a reduction in plasma concentration of IL-1, and an increase in pancreatic DNA synthesis.
Studies have demonstrated that CGRP induces an activation of adenylate cyclase resulting in decreased NF-κB activation and reduced the expression of NF-κB-regulated inflammatory gene products including inflammatory cytokines such as IL-1b and IL-8 and adhesion protein molecules such as ICAM-1 (53). The anti-inflammatory effect of CGRP seems to be mediated by increased anti-inflammatory IL-10 production (54). Above findings suggest that activation of sensory nerves leading to the release of CGRP and other neuropeptides, exerts the anti-inflammatory effect on AP.
Li C et al. (55) have shown that a noxious stimulation of the duodenum can induce pancreatitis via a neurogenic process mediated by TRPA1 and enhanced by high concentrations of ethanol. This data suggested neural cross talk between visceral organs may play a role in mediating inflammation and pain in pancreatitis. A recent study also demonstrated an important finding that TRPV1 and TRPA1 antagonists can prevent the transition of acute to chronic inflammation and pain in CP (56).
Effects of sensory nerves on CP
Effect on generation of pain in CP
CP is a progressive, irreversible inflammatory disease in which pancreatic parenchyma is permanently destroyed and replaced by fibrous tissue, leading to exocrine and endocrine insufficiency, malnutrition and diabetes (57). The most frequent clinical symptom of CP is abdominal pain. The pathogenesis of pain remain unknown, although several possibilities such as increased pancreatic duct and intraparenchymal pressure, pancreatic ischemia and fibrosis and pancreatic calcification have been suggested, more recent studies strongly indicate a major role for alterations in pancreatic sensory nerves (15,58). It has been reported that the mean diameter of nerves, both sensory and motor nerve fibers, are significantly greater in patients with CP than in health people, whereas the mean area of tissue served per nerve is significantly less than in controls, and their severity correlates with the intensity of pain (15). Bockman et al. (15) observed the change of perineurium, which forms a continuous barrier between the nerve and its surroundings, in CP. The absence of the normal barrier provided by the perineurium in CP leads to the nerves subject to the effects of the multitude of products, including activated enzymes in addition to blood plasma components and bioactive materials released from inflammatory cells, present in the connective tissue space of the diseased pancreas and bioactive substances released from eosinophils could cause stimulation of pain. Furthermore it also has been demonstrated that CGRP and SP mediate pancreatic hyperalgesia in CP (59), and their increased immunoreactivity in numerous intrapancreatic nerves (60). Sustained or intense stimulation of sensory nerves results in the release of several neuropeptides, including SP and CGRP, and these neurotransmitters facilitate nociceptive signaling to second order neurons in the spinal cord through via their specific receptors. Investigators have shown both an increase in the expression of SP and CGRP in pancreatic nerves in patients with CP as well as correlation between pain levels and the expression of the neurokinin-1 receptor (60,61).
The fact that total pancreatectomy fails to the alleviation of pain in up to 30% of CP patients (62) supports the view that the chronic pain must in part be sustained by a pancreas-independent mechanism. Recently, evidence from experimental human pain research has illustrated that in many of CP patients, pain processing in the CNS is abnormal (63). In the course of a chronic inflammation of the pancreas, repeated, pathological activation of the afferent pathways carrying visceral information to the brain is likely to cause neuroplastic changes in the peripheral and CNS (64). Experimental studies have manifested that patients with CP show signs of spinal hyperexcitability counter-balanced by segmental and descending inhibition. A recent review showed that an area of the superficial dorsal horn—the substantia gelatinosa—might undergo plastic changes in the setting of chronic pain (65). This area receives inputs from sensory afferents that convey noxious sensation and thus can change synaptic connectivity and receptor expression under certain conditions such as following peripheral tissue damage (65).
Buscher et al. (66) summarized two mechanisms contributing to pain in CP in a previous study: (I) central sensitization and visceral hyperalgesia maintained by persistent nociceptive input from ongoing pancreatic inflammation and (II) autonomous and independent central pain as a result of continuing nociceptive input. It is clear that the peripheral nervous system and CNS undergo plastic changes in response to the inflammation of CP with the result being increased activity in several pain-related nervous system areas. These plastic changes underlie the pathophysiology of chronic pain in CP (64).
So far, various measures for treating pain in CP have included analgesics such as NSAIDs and opioids, and pancreatic enzyme supplements, celiac plexus block, endoscopic techniques and surgery. Kaufman et al. (67) found that endoscopic ultrasound (EUS)-guided celiac plexus block was effective in relieving chronic abdominal pain in 51.46% patients with CP. However, they also noted that improvement in patient selection and refinement of the technique was needed. A systematic review and meta-analysis on the role of EUS-guided celiac plexus block (68) found that celiac plexus block was able to provide pain relief in only 60% of patients.
Effect on chronic pancreatic inflammation and fibrosis
Primary sensory neurons play an important role in pancreatic inflammation in CP and permanent ablation of primary sensory neurons by capsaicin alleviates CP inflammation (69). Several studies have reported that histological alterations of intrapancreatic neural structures are implicated in the development of CP. Previous studies concerning human CP showed that IL-8R positive immune cells were present around the enlarged SP-immunoreactive sensory nerves (70), and that NK-1R immunostaining was seen in the pancreas with inflammatory cells infiltration (61), suggesting that the presence of direct relationship between pancreatic sensory nerves and inflammatory cells as an important mechanism in the pathophysiology of CP. Ikeura T et al. (69) demonstrated that primary sensory nerves denervation by neonatal capsaicin administration attenuated the severity of CP, indicating sensory neurons determined the severity of CP.
SP has been shown to induce lymphocyte proliferation and immunoglobulin synthesis and enhance the release of cytokines from lymphocytes, macrophages, and mast cells, leading to amplification of inflammatory responses (71,72). Furthermore, SP appears to act as a trophic agent in collagen-producing cells and plays an important role in induction of tissue fibrosis in fibrosing disease (73). In addition, NK-1R immunoreactivity is detected in fibroblasts in CP (61), suggests that SP directly affects fibroblasts and promote the development of pancreatic fibrosis.
Conclusions and expectation
The study described in this review shows that sensory nerves play a pivotal role in protecting the pancreas against damage in AP through releasing neurotransmitters from peripheral endings; whereas these nerves promote the development and fibrosis of CP due to the histological alterations of intrapancreatic neural structures. The understanding of the precise effect of sensory nerves on pancreatitis in different phases helps us make distinct therapy for treating pancreatitis. Clinical study has demonstrated that an overall increase in electric and pressure pain thresholds after thoracoscopic splanchnic denervation (TSD) in CP patients (74), indicating that denervation of sensory nerves could be used to pain processing in patients with painful CP. Activation of sensory nerves as one of the methods for treating AP in clinical use could be taken into account.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 1991;43:143-201. [PubMed]
- Buck SH, Burks TF. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev 1986;38:179-226. [PubMed]
- Szallasi A. Vanilloid (capsaicin) receptors in health and disease. Am J Clin Pathol 2002;118:110-21. [PubMed]
- Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion Channel in the pain pathway. Nature 1997;389:816-24. [PubMed]
- Akiba Y, Kato S, Katsube K, et al. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun 2004;321:219-25. [PubMed]
- Ren J, Young RL, Lassiter DC, et al. Calcitonin gene-related peptide mediates capsaicin-induced neuroendocrine responses in rat antrum. Gastroenterology 1993;104:485-91. [PubMed]
- Holzer P, Peskar BM, Peskar BA, et al. Release of calcitonin gene-related peptide induced by capsaicin in the vascularly perfused rat stomach. Neurosci Lett 1990;108:195-200. [PubMed]
- Wimalawansa SJ. The effects of neonatal capsaicin on plasma levels and tissue contents of CGRP. Peptides 1993;14:247-52. [PubMed]
- Dembinski A, Warzecha Z, Konturek PJ, et al. Influence of capsaicin-sensitive afferent neurons and nitric oxide (NO) on cerulein-induced pancreatitis in rats. Int J Pancreatol 1996;19:179-89. [PubMed]
- Warzecha Z, Dembiński A, Jaworek J, et al. Role of sensory nerves in pancreatic secretion and caerulein-induced pancreatitis. J Physiol Pharmacol 1997;48:43-58. [PubMed]
- Dembiński A, Warzecha Z, Ceranowicz P, et al. Stimulation of sensory nerves and CGRP attenuate pancreatic damage in ischemia/reperfusion induced pancreatitis. Med Sci Monit 2003;9:BR418-25. [PubMed]
- Jaworek J, Bonio J, Leja-Szpa A, et al. Sensory nerves in central and peripheral control of pancreatic integrity by leptin and melatonin. J Physiol Pharmacol 2002;53:51-74. [PubMed]
- Salvioli B, Bovara M, Barbara G, et al. Neurology and neuropathology of the pancreatic innervation. JOP 2002;3:26-33. [PubMed]
- Niebergall-Roth E, Singer MV. Central and peripheral neural control of pancreatic exocrine secretion. J Physiol Pharmacol 2001;52:523-38. [PubMed]
- Bockman DE, Buchler M, Malfertheiner P, et al. Analysis of nerves in chronic pancreatitis. Gastroenterology 1988;94:1459-69. [PubMed]
- Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut 2002;51 Suppl 1:i2-5. [PubMed]
- Sengupta JN. An overview of esophageal sensory receptors. Am J Med 2000;108 Suppl 4a:87S-89S. [PubMed]
- Fasanella KE, Christianson JA, Chanthaphavong RS, et al. Distribution and neurochemical identification of pancreatic afferents in the mouse. J Comp Neurol 2008;509:42-52. [PubMed]
- Noble MD, Romac J, Wang Y, et al. Local disruption of the celiac ganglion inhibits substance P release and ameliorates caerulein-induced pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol 2006;291:G128-34. [PubMed]
- Xu GY, Winston JH, Shenoy M, et al. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology 2007;133:1282-92. [PubMed]
- Chang TM, Chey WY. Neurohormonal control of exocrine pancreas. Curr Opin Gastroenterol 2001;17:416-25. [PubMed]
- Konturek SJ, Pepera J, Zabielski K, et al. Brain-gut axis in pancreatic secretion and appetite control. J Physiol Pharmacol 2003;54:293-317. [PubMed]
- Li Y, Hao Y, Owyang C. High-affinity CCK-A receptors on the vagus nerve mediate CCK-stimulated pancreatic secretion in rats. Am J Physiol 1997;273:G679-85. [PubMed]
- Jaworek J, Konturek SJ, Szlachcic A. The role of CGRP and afferent nerves in the modulation of pancreatic enzyme secretion in the rat. Int J Pancreatol 1997;22:137-46. [PubMed]
- Love JA, Yi E, Smith TG. Autonomic pathways regulating pancreatic exocrine secretion. Auton Neurosci 2007;133:19-34. [PubMed]
- Li Y, Wu X, Zhu J, et al. Hypothalamic regulation of pancreatic secretion is mediated by central cholinergic pathways in the rat. J Physiol 2003;552:571-87. [PubMed]
- Peter SA, D’Amato M, Beglinger C. CCK1 antagonists: are they ready for clinical use? Dig Dis 2006;24:70-82. [PubMed]
- Singer MV, Niebergall-Roth E. Secretion from acinar cells of the exocrine pancreas: role of enteropancreatic reflexes and cholecystokinin. Cell Biol Int 2009;33:1-9. [PubMed]
- Chandra R, Liddle RA. Neural and hormonal regulation of pancreatic secretion. Curr Opin Gastroenterol 2009;25:441-6. [PubMed]
- Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology 2004;127:957-69. [PubMed]
- Kirchgessner AL, Gershon MD. Presynaptic inhibition by serotonin of nerve-mediated secretion of pancreatic amylase. Am J Physiol 1995;268:G339-45. [PubMed]
- Li Y, Wu XY, Zhu JX, et al. Intestinal serotonin acts as paracrine substance to mediate pancreatic secretion stimulated by luminal factors. Am J Physiol Gastrointest Liver Physiol 2001;281:G916-23. [PubMed]
- Nawrot-Porabka K, Jaworek J, Leja-Szpak A, et al. Involvement of vagal nerves in the pancreatostimulatory effects of luminal melatonin, or its precursor L-tryptophan. Study in the rats. J Physiol Pharmacol 2007;58:81-95. [PubMed]
- Sabbatini ME. Natriuretic peptides as regulatory mediators of secretory activity in the digestive system. Regul Pept 2009;154:5-15. [PubMed]
- Sabbatini ME, Rodríguez MR, Dabas P, et al. C-type natriuretic peptide stimulates pancreatic exocrine secretion in the rat: role of vagal afferent and efferent pathways. Eur J Pharmacol 2007;577:192-202. [PubMed]
- Jaworek J, Nawrot-Porabka K, Leja-Szpak A, et al. Brain-gut axis in the modulation of pancreatic enzyme secretion. J Physiol Pharmacol 2010;61:523-31. [PubMed]
- Menger MD, Plusczyk T, Vollmar B. Microcirculatory derangements in acute pancreatitis. J Hepatobiliary Pancreat Surg 2001;8:187-94. [PubMed]
- Klar E, Schratt W, Foitzik T, et al. Impact of microcirculatory flow pattern changes on the development of acute edematous and necrotizing pancreatitis in rabbit pancreas. Dig Dis Sci 1994;39:2639-44. [PubMed]
- Chen HM, Sunamura M, Shibuya K, et al. Early microcirculatory derangement in mild and severe pancreatitis models in mice. Surg Today 2001;31:634-42. [PubMed]
- Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg 2006;93:518-30. [PubMed]
- Hackert T, Pfeil D, Hartwig W, et al. Platelet function in acute experimental pancreatitis induced by ischaemia-reperfusion. Br J Surg 2005;92:724-8. [PubMed]
- Warzecha Z, Dembiński A, Ceranowicz P, et al. Protective effect of calcitonin gene-related peptide against caerulein-induced pancreatitis in rats. J Physiol Pharmacol 1997;48:775-87. [PubMed]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 2004;84:903-34. [PubMed]
- Warzecha Z, Dembiński A, Ceranowicz P, et al. Calcitonin gene-related peptide can attenuate or augment pancreatic damage in caerulein-induced pancreatitis in rats. J Physiol Pharmacol 1999;50:49-62. [PubMed]
- Warzecha Z, Dembiński A, Ceranowicz P, et al. Effect of sensory nerves and CGRP on the development of caerulein-induced pancreatitis and pancreatic recovery. J Physiol Pharmacol 2001;52:679-704. [PubMed]
- Liddle RA, Nathan JD. Neurogenic inflammation and pancreatitis. Pancreatology 2004;4:551-9; discussion 559-60. [PubMed]
- Liddle RA. The role of Transient Receptor Potential Vanilloid 1 (TRPV1) channels in pancreatitis. Biochim Biophys Acta 2007;1772:869-78.
- Harada N, Okajima K, Kurihara H, et al. Stimulation of sensory neurons by capsaicin increases tissue levels of IGF-I, thereby reducing reperfusion-induced apoptosis in mice. Neuropharmacology 2007;52:1303-11. [PubMed]
- Rongione AJ, Kusske AM, Reber HA, et al. Interleukin-10 reduces circulating levels of serum cytokines in experimental pancreatitis. J Gastrointest Surg 2006;1:159-65;discussion165-6.
- Jaworek J, Leja-Szpak A, Dembiński A, et al. Involvement of sensory nerves in the protective effect of growth hormone on acute pancreatitis. Growth Horm IGF Res 2009;19:517-22. [PubMed]
- Schneider L, Hackert T, Heck M, et al. Capsaicin reduces tissue damage in experimental acute pancreatitis. Pancreas 2009;38:676-80. [PubMed]
- Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood 1991;77:1627-52. [PubMed]
- Schneider L, Hartwig W, Flemming T, et al. Protective effects and anti-inflammatory pathways of exogenous calcitonin gene-related peptide in severe necrotizing pancreatitis. Pancreatology 2009;9:662-9. [PubMed]
- Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock 2005;24 Suppl 1:45-51. [PubMed]
- Li C, Zhu Y, Shenoy M, et al. Anatomical and functional characterization of a duodeno-pancreatic neural reflex that can induce acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 2013;304:G490-500. [PubMed]
- Schwartz ES, La JH, Scheff NN, et al. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci 2013;33:5603-11. [PubMed]
- Braganza JM, Lee SH, McCloy RF, et al. Chronic pancreatitis. Lancet 2011;377:1184-97. [PubMed]
- Anaparthy R, Pasricha PJ. Pain and chronic pancreatitis: is it the plumbing or the wiring? Curr Gastroenterol Rep 2008;10:101-6. [PubMed]
- Liu L, Shenoy M, Pasricha PJ. Substance P and calcitonin gene related peptide mediate pain in chronic pancreatitis and their expression is driven by nerve growth factor. JOP 2011;12:389-94. [PubMed]
- Büchler M, Weihe E, Friess H, et al. Changes in peptidergic innervation in chronic pancreatitis. Pancreas 1992;7:183-92. [PubMed]
- Shrikhande SV, Friess H, di Mola FF, et al. NK-1 receptor gene expression is related to pain in chronic pancreatitis. Pain 2001;91:209-17. [PubMed]
- Rattner DW, Fernandez-del Castillo C, Warshaw AL. Pitfalls of distal pancreatectomy for relief of pain in chronic pancreatitis. Am J Surg 1996;171:142-5; discussion 145-6. [PubMed]
- Drewes AM, Krarup AL, Detlefsen S, et al. Pain in chronic pancreatitis: the role of neuropathic pain mechanisms. Gut 2008;57:1616-27. [PubMed]
- Fregni F, Pascual-Leone A, Freedman SD. Pain in chronic pancreatitis: a salutogenic mechanism or a maladaptive brain response? Pancreatology 2007;7:411-22. [PubMed]
- Furue H, Katafuchi T, Yoshimura M. Sensory processing and functional reorganization of sensory transmission under pathological conditions in the spinal dorsal Horn. Neurosci Res 2004;48:361-8. [PubMed]
- Buscher HC, Wilder-Smith OH, van Goor H. Chronic pancreatitis patients show hyperalgesia of central origin: a pilot study. Eur J Pain 2006;10:363-70. [PubMed]
- Kaufman M, Singh G, Das S, et al. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol 2010;44:127-34. [PubMed]
- Puli SR, Reddy JB, Bechtold ML, et al. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci 2009;54:2330-7. [PubMed]
- Ikeura T, Kataoka Y, Wakabayashi T, et al. Effects of sensory denervation by neonatal capsaicin administration on experimental pancreatitis induced by dibutyltin dichloride. Med Mol Morphol 2007;40:141-9. [PubMed]
- Di Sebastiano P, di Mola FF, Di Febbo C, et al. Expression of interleukin 8 (IL-8) and substance P in human chronic pancreatitis. Gut 2000;47:423-8. [PubMed]
- Ho WZ, Kaufman D, Uvaydova M, et al. Substance P augments interleukin-10 and tumor necrosis factor-alpha release by human cord blood monocytes and macrophages. J Neuroimmunol 1996;71:73-80. [PubMed]
- Maggi CA. The effects of tachykinins on inflammatory and immune cells. Regul Pept 1997;70:75-90. [PubMed]
- Katayama I, Nishioka K. Substance P augments fibrogenic cytokine-induced fibroblast proliferation: possible involvement of neuropeptide in tissue fibrosis. J Dermatol Sci 1997;15:201-6. [PubMed]
- Buscher HC, van Goor H, Wilder-Smith OH. Effect of thoracoscopic splanchnic denervation on pain processing in chronic pancreatitis patients. Eur J Pain 2007;11:437-43. [PubMed]


