The pros and cons of prophylactic central neck dissection in papillary thyroid carcinoma
Introduction
Compared to many malignancies, most patients diagnosed with differentiated thyroid carcinomas have an excellent prognosis, with five-year overall survival of well over 90% (1,2). However, recurrence following treatment is common, occurring in up to 30% of patients and can occur up-to 20 years after the initial diagnosis (3-5). Recurrence most commonly occurs in the cervical lymph nodes and is generally managed with surgical resection (6). Prophylactic central neck dissection (pCND) is performed to remove occult nodal metastases that are not detected by clinical examination or pre-operative radiological assessment. pCND offers the possibility of reducing the risk of central neck recurrence by removing central compartment nodal tissue during the initial thyroidectomy; thereby reducing the need for re-operative surgery in the central neck with the attendant risks of injury to the recurrent laryngeal nerves and parathyroid glands. It should be noted that a pCND is not devoid of risk and still carries a risk of nerve and parathyroid morbidity itself. The indications and role of pCND therefore remain a controversial area of thyroid cancer management (7).
This review will focus on pCND for papillary thyroid cancer. The evidence and arguments for and against pCND, within the context of long-term outcomes and quality of life (QoL), will be discussed (Table 1).
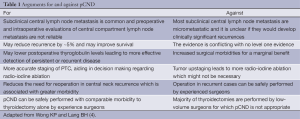
Full table
Papillary thyroid carcinoma (PTC) and nodal metastasis
Approximately 85% of differentiated thyroid cancers are PTC. Up to 35% of PTC patients present with macrometastatic cervical lymph node metastasis, and up to 80% will possess undetectable, microscopic cervical lymph node disease (8-10). The presence of macrometastatic nodal metastasis is an independent predictor for recurrence, while the significance of micrometastatic disease is controversial and may have little prognostic significance (6,11,12). Due to this high rate of lymph node metastasis, preoperative cervical ultrasound with fine needle aspiration biopsy of suspicious lymph nodes and therapeutic neck dissection for patients with confirmed metastatic lymph nodes is recommended treatment (7,13).
While ultrasound is a sensitive and specific test for detecting lateral cervical lymph node metastasis, diagnosing central lymph node metastasis is much more difficult. Central lymph nodes are positioned over the trachea and closely located to the thyroid making identification of nodal metastasis problematic (14). Pre-operative imaging with ultrasound or computerised tomography have limited sensitivity at detecting metastatic central lymph nodes. Ultrasound has a sensitivity of 53-61% with a specificity of 80-93%, whilst computerised tomography has been found to have a sensitivity of 67% with a specificity of 79-91% (14). Positron emission tomography is even more limited in detecting central lymph node metastasis pre-operatively with sensitivities of less than 40% reported (15,16). Given these findings, radiological investigations cannot be relied on to accurately detect most central lymph node metastasis pre-operatively. As the incidence of metastasis in the central lymph nodes is estimated to be between 30-70% (10,17,18), and due to the fact that the central lymph nodes can be accessed during, and through the same incision as for thyroidectomy, it has been standard practice in a number of specialist units to clear the central lymph nodes during the initial thyroidectomy.
Clearance of the central lymph nodes requires identification and preservation of the recurrent laryngeal nerves and avoidance of parathyroid devascularisation. The potential risks of nerve injury and permanent hypoparathyroidism using this approach must be carefully considered against the benefit of prophylactic nodal dissection.
Definitions
Due to the controversy and number of publications regarding central neck dissection (CND), it is important to employ agreed definitions for discussion. The American Thyroid Association Surgery Working Group has defined the anatomy and surgical terminology as follows:
- The central compartment (level VI) of the neck is described anatomically as being bound superiorly by the hyoid bone, laterally by the carotid arteries, anteriorly by the superficial layer of the deep cervical fascia and posteriorly by the deep layer of the deep cervical fascia, inferiorly by the innominate artery on the right and corresponding axial plane on the left. Included in this definition of the central neck is the anterior superior mediastinum above the innominate artery (level VII);
- CND is defined as a comprehensive, compartment-orientated removal of the prelaryngeal and pretracheal nodes and at least one paratracheal basin;
- CND can be bilateral or unilateral. Bilateral CND removes both the right and left para-tracheal nodes along with the pre-laryngeal and pre-tracheal nodes. Unilateral CND removes one side of the para-tracheal nodes along with the pre-laryngeal and pre-tracheal lymph nodes. It is important to note that CND is a compartmental clearance of lymph nodes and does not include ‘berry picking’ of selected lymph nodes within the central compartment;
- Lymph node dissection can be therapeutic: where patients have clinically or radiologically detectable lymph node disease prior to surgery; or prophylactic: where lymph node metastasis is not identified pre-operatively (19).
A further important point relates to the definition of micro- vs. macro-metastatic disease. In the literature, the definition of macro-metastatic ranges from greater than 2 mm, to over 10 mm (10,12,20). The definition of micro-metastatic disease is controversial with the limit of less than 2 mm being adapted from the description from breast cancer metastasis and used for most studies of pCND (11). An accepted definition is important as micro-metastatic disease has a significantly lower risk of recurrence compared to macro-metastatic disease (10-12).
Significance of lymph node metastasis in PTC
Unlike most malignancies where nodal metastasis correlates with more advanced disease and a substantially worse prognosis, PTC related nodal metastases have traditionally been considered to have little impact on recurrence or survival outcomes. This is typified by numerous prognostic scoring systems such as the MACIS (Metastasis, Age, Completeness of Resection, Invasion, Size) and European Organization for Research and Treatment of Cancer (EORTC) which do not include nodal metastasis as a prognostic indicator.
Despite this conventional reasoning, large scale SEER database analyses have shown that lymph node metastasis is a significant predictor of overall survival (21). This is reflected in the American Joint Committee on Cancer (AJCC) Tumour, Nodal disease and distant Metastasis (TNM) staging system which classifies lymph node metastasis as a prognostic factor for patients aged over 45 years and can have a marked impact on staging of disease. For instance, according to the TNM system, lymph node metastasis (in a patient aged older than 45 years) moves the stage from I to III (22). However the TNM classification does not differentiate between micro- or macro-metastatic lymph nodes and does not sub-classify lymph node disease between the lateral and central cervical compartments.
The presence of lymph node metastasis is also an independent risk factor for recurrence (23). To predict the risk of recurrence it is important to consider a number of factors. A spectrum of recurrence risk is seen from 4% to 34%, depending on the primary tumour characteristics, patient age and number of metastatic nodes (12,24). The risk of recurrence has also been shown to relate to the specific burden of lymph node metastasis, with recurrence rates of 32% and 5% when comparing macro- (≥2 mm) versus micro-metastasis respectively (11). The use of lymph node ratio also has potential to classify recurrence risk, with a high lymph node ratio (defined as being >40% of metastatic to total resected nodes) being found to be an independent predictor of recurrence on multivariate analysis (20). The use of lymph node ratios based on central compartment dissection may also facilitate tailored management plans (25). A weakness of using lymph node ratio is the extent of central lymph node dissection. If only abnormal lymph nodes are removed and the central compartment not cleared as recommend by guidelines it will result in a spuriously elevated lymph node ratio, limiting its use as a prognostic system.
For unilateral PTC, the location of central lymph node metastasis is predominantly ipsilateral with less than 10% involving the contralateral central lymph nodes (26,27). Multi-focal PTCs and cancers of the isthmus are more likely to have contralateral nodal metastasis in the central compartment.
Loco-regional control with pCND
The central argument employed to justify pCND relates to the assertion that it reduces the rate of central neck recurrence and thereby minimises the need for re-operative surgery. This argument is controversial, and has been questioned on the basis of largely retrospective studies justifying its utility. Until recently, no studies had shown a significant reduction in the rate of central lymph node recurrence following pCND (28). A number of meta-analyses of retrospective cohort series have also not shown any beneficial reduction in recurrence when comparing the use of pCND and total thyroidectomy to thyroidectomy alone (29,30).
Conversely, a recent series by Barczyński et al. with an appropriate follow-up period has shown that pCND can lead to a significantly reduced loco-regional recurrence rate. This cohort study with a median follow up of 120 months, demonstrated that locoregional control is significantly better with pCND (6.9% difference seen at 10 years; P=0.003) (31). These results were supported by Popadich et al. who showed a significant 4.6% reduction in central compartment recurrence requiring re-operation when compared to patients undergoing total thyroidectomy alone across three specialised endocrine surgery units (P=0.004) (32). A subsequent meta-analysis (including these recent series), has shown that by restricting prophylactic resection to adults with PTCs >1 cm there is a trend towards lower recurrence rates; with a number needed to treat of 31 patients (to avoid one recurrence) (33).
Conflicting evidence regarding the benefit of pCND on outcomes such as recurrence are likely to continue. Given the small differences observed between those treated with and without pCND, a randomised controlled trial is not feasible from both a power analysis and economic viewpoint (34).
Beyond recurrence rates, defining any difference in survival with pCND is also difficult owing to the typically good prognosis that the majority of PTC patients enjoy. Despite these challenges, Barczyński et al. has previously shown that patients treated with bilateral pCND had significantly improved survival compared to a historical control group of patients treated with total thyroidectomy alone, with ten-year disease-specific survival improved from 92.5% to 98.0% (31). A significant criticism of this study involved the increased use of radioactive iodine (RAI) in the pCND group due to the information gained from lymph node staging, which may have affected disease specific survival. Whilst potentially confounding outcome data, it could be argued that improved outcomes would not have been obtained without this additional prognostic information; further highlighting the prophylactic, as opposed to purely therapeutic benefit of pCND.
Staging and RAI ablation
As discussed, an additional argument in favour of pCND is the staging information it provides, which can guide use of RAI (131I). A substantial number of patients undergoing pCND may be upstaged, allowing for more stage appropriate post-operative RAI ablation therapy (35). This upstaging effect was typified by Hughes et al., who showed that for patients aged over 45, one third were upstaged due to lymph node metastasis detected by pCND and recommended for RAI (17). Staging information obtained from pCND can be used to personalise the use of RAI. This approach has been shown by Lang et al. (36), who has shown the utility of using size of the central lymph node metastases to predict the response to RAI ablation. In this study, patients with macrometastatic lymph node disease (≥2 mm) were found to be six times more likely to have detectable stimulated thyroglobulin nine months after surgery, leading the authors to suggest that the RAI dose could be tailored according to the size of metastatic disease (36).
Staging information obtained from pCND is of particular use when deciding if patients with PTCs of 1-2 cm should undergo RAI, as it is selectively recommended in this scenario (13). Lymph node involvement from pCND has been shown to be an indication for treatment in up to 30% of these patients allowing for a more tailored approach to treatment (37). The additional benefit of this approach for patients staged as having no nodal metastasis (pN0) after CLND involves being able to safely receive lower doses of 131I or not receive it at all (37). This approach may also lead to an increase in the number of patients found to have a negative low-dose total body scan, thereby reducing the number of patients who require RAI ablation (7).
Conversely, the potential upstaging from pCND can lead to overtreatment with RAI. When making decisions regarding RAI based on lymph node metastasis it is essential that the factors that predict increased risk of recurrence such as macro-metastatic lymph node disease are considered (28). Treatment with RAI carries morbidity risks, such as salivary and lacrimal gland dysfunction, dysphagia and an increased risk of secondary malignancies (28).
In addition to providing information used to tailor RAI treatment, staging information from pCND can facilitate follow-up protocols, allowing for closer follow-up for patients deemed at higher risk of recurrence on the basis of positive central compartment nodes (38).
Thyroglobulin normalization
A treatment goal of PTC is to facilitate long-term surveillance for PTC recurrence, for which thyroglobulin levels are used as a marker of persistent and recurrent disease (13). Advocates for pCND argue that by removing subclinical metastases, postoperative serum thyroglobulin will be lower and assists in achieving the goal of athyroglobulinemia. This suggestion would appear to make theoretical sense and has been justified in two studies comparing pCND to no pCND (31,39). However, other units have failed to demonstrate differences in thyroglobulin levels between similar patient groups (17). The ability of pCND to increase the rate of athyroglobulinemia and the long-term prognostic and management significance of this remain to be evaluated in further studies.
Re-operative surgery following CND
An argument against pCND is that secondary operation for lymph node recurrence can be performed safely in the central neck compartment by experienced thyroid surgeons with similar morbidity seen with pCND (40-42). This statement is controversial however with other studies reporting higher rates of hypoparathyroidism and vocal cord paralysis with re-operative surgery, due to tumour recurrence and local invasion (43).
The diagnosis of recurrence and the requirement for re-operation is also highly significant to the patient and treating clinician. It could be argued that such considerable anxiety may be avoided if the central lymph nodes are cleared at initial surgery (7). Furthermore it has been shown that patients who experience recurrence are at increased risk for subsequent recurrence, with a number of patients needing multiple operations (44,45). Re-operative surgery is less likely to achieve a biochemical cure of disease with a minority of patients (27%) achieving undetectable serum thyroglobulin levels post-operatively (46,47). Evans has used these findings to justify his assertion that ‘the best chance to remove all disease is at the first operation’ (7).
Complications of pCND
Given the contentious benefits of pCND, the risk of pCND related complications are of critical importance and are the most serious disadvantage of offering pCND. The risks of pCND are the same as at thyroidectomy and include recurrent laryngeal nerve injury, hypoparathyroidism and hematoma. For total thyroidectomy, permanent hypoparathyroidism and nerve injury rates less than 1-2% have been suggested as being acceptable for experienced surgeons (48).
With regard to the addition of pCND to total thyroidectomy, multiple studies have shown a significantly increased risk of temporary hypoparathyroidism, presumably due to the vasculature of the parathyroids being at risk of manipulation and division during dissection (28,32,49). Whilst surgery by a specialist unit can minimise permanent complications to similar levels as seen with total thyroidectomy alone, multiple studies have shown a trend towards increased permanent hypoparathyroidism and recurrent laryngeal nerve injury when comparing total thyroidectomy with pCND to thyroidectomy alone (28).
The choice between unilateral and bilateral pCND is also important and it could be assumed that a bilateral dissection would be associated with a higher complication rate. This has been borne out by the works of Giordano et al., who showed in a retrospective analysis of 1,087 patients that ipsilateral pCND caused significantly less permanent hypoparathyroidism when compared to bilateral pCND (7% vs. 16.2%, P<0.001) (50).
The morbidity and potential threat to life of recurrent laryngeal nerve palsy and subsequent vocal cord paralysis has been known since the times of Galen in the second century A.D. and do not require additional discussion (51). Permanent hypoparathyroidism can also cause considerable morbidity due to the requirement for calcium replacement and monitoring with the need to avoid hypercalcemia, nephrocalcinosis and renal failure. Rates of chronic kidney disease in patients with permanent hypoparathyroidism have been estimated at 2- to 17-fold greater than age adjusted controls (52). Surgery in a specialist unit with liberal use of parathyroid autotransplantion and replacement of calcium post-operatively with high dosages of oral calcium and vitamin D has been suggested as an important strategy to prevent permanent hypoparathyroidism (31,39,53).
In general, complications can be limited by careful attention to operative technique. During dissection of the central lymph nodes, it is essential to have clear visualisation of the recurrent laryngeal nerve throughout its cervical course. When in close proximity to the recurrent laryngeal nerve, sharp dissection should be used instead of electrocautery to minimise the chance of lateral thermal spread and injury (39).
Due to the possibility of significant morbidity, it is essential that pCND is offered by units with large thyroid workloads, who regularly review complication rates (49). pCND in experienced thyroid units is able to be performed with no significant increase in the rates of permanent complications (32).
QoL studies in thyroid cancer
Long term survival rates, the nature of treatment with initial surgery, subsequent RAI ablation and the need for long-term monitoring impose significant challenges on thyroid cancer survivors and can affect health-related QoL (54). QoL research in PTC has been restricted due to the lack of a specific thyroid cancer model (55). However, with the advent of a thyroid cancer disease specific health related quality of life questionnaire (THYCA-QoL) it is hoped that this area of thyroid cancer research will develop and provide further evidence to support best practice (56). The 24 questions identified in this survey include many that are relevant to pCND, including symptoms of hypoparathyroidism, vocal cord palsy and recurrence. Furthermore, symptoms related to hypoparathyroidism have been found to be significantly increased in thyroid cancer survivors (57). These findings are important for clinicians to discuss with patients when considering pCND.
Routine versus selective pCND and the importance of specialized surgical practice
Table 2 presents a summary of the recommendations for pCND in PTC. pCND is increasingly being recommended only for those with a higher risk of recurrence (63). However, some prognostic features used to distinguish those at a higher recurrence risk (such as aggressive variants and extrathyroidal extension) can be difficult to diagnose pre-operatively making selection of patients difficult.
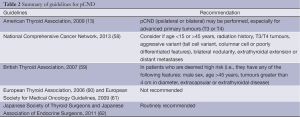
Full table
Population studies suggest that those who would gain the most benefit (e.g., older patients) are not undergoing pCND as regularly as lower risk patients and the adequacy of surgery as defined by lymph node retrieval patterns varies widely (64,65). Some units recommend the selection of patients for pCND by use of intra-operative factors, such as frozen section analysis of lymph nodes (66). The practice of the authors’ unit (University of Sydney Endocrine Surgery Unit) is to offer routine ipsilateral pCND for patients with PTCs >1 cm. This unit is able to practice pCND with similar outcomes to total thyroidectomy alone with a technique involving full visualisation and protection of the recurrent laryngeal nerves and liberal use of autotransplantion of the inferior parathyroids (67).
Whether routine or selective pCND is practiced, it should only be offered by units with experience in thyroid surgery. It is well recognized that the complications of thyroid surgery, especially recurrent laryngeal nerve injury and hypo-parathyroidism are higher in low volume units (63,68). As these complications are the same with pCND and given the potential benefit of pCND can be small it is essential that the risks of complications be minimized. pCND cannot be recommended for low volume surgeons and patients should be referred to specialist thyroid surgeons performing at least 50 endocrine operations annually and with experience in central lymph node dissection (63).
Future treatment strategies
Advances in knowledge regarding the underlying molecular pathogenesis of PTC offers potential novel stratification tools that may be employed to select patients who would most benefit from pCND (69). B-type Raf kinase (BRAFV600E) mutation has been associated with aggressive disease and loss of RAI avidity in recurrent disease. The ability to diagnose the mutation on pre-operative biopsy means it could be a useful tool for selecting patients who would most benefit from pCND (69). Alzahrani and Xing have shown that BRAFV600E mutation is associated with high-risk characteristics of PTC such as extrathyroidal extension and could be used as an indication for CND (70). In addition, Howell et al. has shown that BRAFV600E mutation is an independent predictor of central lymph node metastasis (71). The use of translational molecular markers offers great promise, however this strategy remains to be confirmed in clinical trials.
The role of sentinel node biopsy is also under investigation and is currently used in some treatment centres, however given the high rates of lymph node metastasis with PTC it may not be an effective operative strategy in selecting patients for pCND (72).
Conclusions
The successful treatment of PTC requires careful consideration by the patient and clinician of the potential benefits and morbidity of each treatment modality. While PTC has high overall survival, recurrence is common and pCND has a potentially significant role in the management of PTC if it can be offered with minimal morbidity. Morbidity from pCND is directly related to surgical experience, and its use must be carefully evaluated by patients and clinicians depending on local resources. The potential use of molecular markers will hopefully offer a further strategy to stratify the risk of recurrence with PTC and allow a more tailored approach to offer pCND to patients with the greatest benefit.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A.. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev 2009;18:784-91. [PubMed]
- Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940-1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 2002;26:879-85. [PubMed]
- Wong KP, Lang BH. The role of prophylactic central neck dissection in differentiated thyroid carcinoma: issues and controversies. J Oncol 2011;2011:127929.
- Sippel RS, Chen H. Controversies in the surgical management of newly diagnosed and recurrent/residual thyroid cancer. Thyroid 2009;19:1373-80. [PubMed]
- Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994;97:418-28. [PubMed]
- Evans DB. Papillary carcinoma of the thyroid: balancing principles of oncology with emerging technology. Surgery 2011;150:1015-22. [PubMed]
- Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 Cancer 1998;83:2638-48. [PubMed]
- Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery 2003;134:946-54; discussion 954-5. [PubMed]
- Clark OH. Thyroid cancer and lymph node metastases. J Surg Oncol 2011;103:615-8. [PubMed]
- Cranshaw IM, Carnaille B. Micrometastases in thyroid cancer. An important finding? Surg Oncol 2008;17:253-8. [PubMed]
- Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012;22:1144-52. [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [PubMed]
- Mulla M, Schulte KM. Central cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the central compartment. Clin Endocrinol (Oxf) 2012;76:131-6. [PubMed]
- Kim BS, Ryu HS, Kang KH. The value of preoperative PET-CT in papillary thyroid cancer. J Int Med Res 2013;41:445-56. [PubMed]
- Jeong HS, Baek CH, Son YI, et al. Integrated 18F-FDG PET/CT for the initial evaluation of cervical node level of patients with papillary thyroid carcinoma: comparison with ultrasound and contrast-enhanced CT. Clin Endocrinol (Oxf) 2006;65:402-7. [PubMed]
- Hughes DT, White ML, Miller BS, et al. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery 2010;148:1100-6; discussion 1006-7. [PubMed]
- Takada H, Kikumori T, Imai T, et al. Patterns of lymph node metastases in papillary thyroid carcinoma: results from consecutive bilateral cervical lymph node dissection. World J Surg 2011;35:1560-6. [PubMed]
- American Thyroid Association Surgery Working Group, American Association of Endocrine Surgeons, American Academy of Otolaryngology-Head and Neck Surgery, et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid 2009;19:1153-8. [PubMed]
- Jeon MJ, Yoon JH, Han JM, et al. The prognostic value of the metastatic lymph node ratio and maximal metastatic tumor size in pathological N1a papillary thyroid carcinoma. Eur J Endocrinol 2013;168:219-25. [PubMed]
- Podnos YD, Smith D, Wagman LD, et al. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg 2005;71:731-4. [PubMed]
- Cancer AJCO. AJCC Cancer Staging Manual. 7 ed. In: Edge SB, Byrd DR, Compton CC, et al. eds. Springer, 2010.
- Mazzaferri EL, Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med 1981;70:511-8. [PubMed]
- Ducoudray R, Trésallet C, Godiris-Petit G, et al. Prophylactic lymph node dissection in papillary thyroid carcinoma: is there a place for lateral neck dissection? World J Surg 2013;37:1584-91. [PubMed]
- Schneider DF, Chen H, Sippel RS. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol 2013;20:1906-11. [PubMed]
- Roh JL, Kim JM, Park CI. Central lymph node metastasis of unilateral papillary thyroid carcinoma: patterns and factors predictive of nodal metastasis, morbidity, and recurrence. Ann Surg Oncol 2011;18:2245-50. [PubMed]
- Nam IC, Park JO, Joo YH, et al. Pattern and predictive factors of regional lymph node metastasis in papillary thyroid carcinoma: a prospective study. Head Neck 2013;35:40-5. [PubMed]
- Gyorki DE, Untch B, Tuttle RM, et al. Prophylactic central neck dissection in differentiated thyroid cancer: an assessment of the evidence. Ann Surg Oncol 2013;20:2285-9. [PubMed]
- Zetoune T, Keutgen X, Buitrago D, et al. Prophylactic central neck dissection and local recurrence in papillary thyroid cancer: a meta-analysis. Ann Surg Oncol 2010;17:3287-93. [PubMed]
- Shan CX, Zhang W, Jiang DZ, et al. Routine central neck dissection in differentiated thyroid carcinoma: a systematic review and meta-analysis. Laryngoscope 2012;122:797-804. [PubMed]
- Barczyński M, Konturek A, Stopa M, et al. Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg 2013;100:410-8. [PubMed]
- Popadich A, Levin O, Lee JC, et al. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery 2011;150:1048-57. [PubMed]
- Wang TS, Cheung K, Farrokhyar F, et al. A meta-analysis of the effect of prophylactic central compartment neck dissection on locoregional recurrence rates in patients with papillary thyroid cancer. Ann Surg Oncol 2013;20:3477-83. [PubMed]
- Carling T, Carty SE, Ciarleglio MM, et al. American Thyroid Association design and feasibility of a prospective randomized controlled trial of prophylactic central lymph node dissection for papillary thyroid carcinoma. Thyroid 2012;22:237-44. [PubMed]
- Shindo M, Wu JC, Park EE, et al. The importance of central compartment elective lymph node excision in the staging and treatment of papillary thyroid cancer. Arch Otolaryngol Head Neck Surg 2006;132:650-4. [PubMed]
- Lang BH, Tang AH, Wong KP, et al. Significance of size of lymph node metastasis on postsurgical stimulated thyroglobulin levels after prophylactic unilateral central neck dissection in papillary thyroid carcinoma. Ann Surg Oncol 2012;19:3472-8. [PubMed]
- Bonnet S, Hartl D, Leboulleux S, et al. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab 2009;94:1162-7. [PubMed]
- Hartl DM, Leboulleux S, Al Ghuzlan A, et al. Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann Surg 2012;255:777-83. [PubMed]
- Sywak M, Cornford L, Roach P, et al. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery 2006;140:1000-5; discussion 1005-7. [PubMed]
- Shen WT, Ogawa L, Ruan D, et al. Central neck lymph node dissection for papillary thyroid cancer: comparison of complication and recurrence rates in 295 initial dissections and reoperations. Arch Surg 2010;145:272-5. [PubMed]
- Alvarado R, Sywak MS, Delbridge L, et al. Central lymph node dissection as a secondary procedure for papillary thyroid cancer: Is there added morbidity? Surgery 2009;145:514-8. [PubMed]
- Schuff KG, Weber SM, Givi B, et al. Efficacy of nodal dissection for treatment of persistent/recurrent papillary thyroid cancer. Laryngoscope 2008;118:768-75. [PubMed]
- Roh JL, Kim JM, Park CI. Central compartment reoperation for recurrent/persistent differentiated thyroid cancer: patterns of recurrence, morbidity, and prediction of postoperative hypocalcemia. Ann Surg Oncol 2011;18:1312-8. [PubMed]
- Clayman GL, Agarwal G, Edeiken BS, et al. Long-term outcome of comprehensive central compartment dissection in patients with recurrent/persistent papillary thyroid carcinoma. Thyroid 2011;21:1309-16. [PubMed]
- Marshall CL, Lee JE, Xing Y, et al. Routine pre-operative ultrasonography for papillary thyroid cancer: effects on cervical recurrence. Surgery 2009;146:1063-72. [PubMed]
- Steward DL. Update in utility of secondary node dissection for papillary thyroid cancer. J Clin Endocrinol Metab 2012;97:3393-8. [PubMed]
- Al-Saif O, Farrar WB, Bloomston M, et al. Long-term efficacy of lymph node reoperation for persistent papillary thyroid cancer. J Clin Endocrinol Metab 2010;95:2187-94. [PubMed]
- Mazzaferri EL, Doherty GM, Steward DL. The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid 2009;19:683-9. [PubMed]
- Chisholm EJ, Kulinskaya E, Tolley NS. Systematic review and meta-analysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone. Laryngoscope 2009;119:1135-9. [PubMed]
- Giordano D, Valcavi R, Thompson GB, et al. Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid 2012;22:911-7. [PubMed]
- Kaplan EL, Salti GI, Roncella M, et al. History of the recurrent laryngeal nerve: from Galen to Lahey. World J Surg 2009;33:386-93. [PubMed]
- Mitchell DM, Regan S, Cooley MR, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab 2012;97:4507-14. [PubMed]
- Sitges-Serra A, Ruiz S, Girvent M, et al. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg 2010;97:1687-95. [PubMed]
- Singer S, Lincke T, Gamper E, et al. Quality of life in patients with thyroid cancer compared with the general population. Thyroid 2012;22:117-24. [PubMed]
- Lee JI, Kim SH, Tan AH, et al. Decreased health-related quality of life in disease-free survivors of differentiated thyroid cancer in Korea. Health Qual Life Outcomes 2010;8:101. [PubMed]
- Husson O, Haak HR, Mols F, et al. Development of a disease-specific health-related quality of life questionnaire (THYCA-QoL) for thyroid cancer survivors. Acta Oncol 2013;52:447-54. [PubMed]
- Husson O, Haak HR, Buffart LM, et al. Health-related quality of life and disease specific symptoms in long-term thyroid cancer survivors: a study from the population-based PROFILES registry. Acta Oncol 2013;52:249-58. [PubMed]
- Thyroid Carcinoma. 2nd ed. NCCN Guidelines; 2010:1-104. Available online: http://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf, accessed 25/8/2013
- Association BT. Guidelines for the Management of Thyroid Cancer - British Thyroid Association - Google Books. 2007.
- Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 2006;154:787-803. [PubMed]
- Pacini F, Castagna MG, Brilli L, et al. Differentiated thyroid cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20 Suppl 4:143-6. [PubMed]
- Takami H, Ito Y, Okamoto T, et al. Therapeutic strategy for differentiated thyroid carcinoma in Japan based on a newly established guideline managed by Japanese Society of Thyroid Surgeons and Japanese Association of Endocrine Surgeons. World J Surg 2011;35:111-21. [PubMed]
- Stavrakis AI, Ituarte PH, Ko CY, et al. Surgeon volume as a predictor of outcomes in inpatient and outpatient endocrine surgery. Surgery 2007;142:887-99; discussion 887-99. [PubMed]
- Enyioha C, Roman SA, Sosa JA. Central lymph node dissection in patients with papillary thyroid cancer: a population level analysis of 14,257 cases. Am J Surg 2013;205:655-61. [PubMed]
- Lang BH, Yih PC, Shek TW, et al. Factors affecting the adequacy of lymph node yield in prophylactic unilateral central neck dissection for papillary thyroid carcinoma. J Surg Oncol 2012;106:966-71. [PubMed]
- Shaha AR. Controversies about the central compartment in thyroid cancer. Editorial regarding the article “Clinical impact of cervical lymph node involvement and central neck dissection in patients with papillary thyroid carcinoma: a retrospective analysis of 368 cases” by Alexandre Bozec et al. Eur Arch Otorhinolaryngol 2011;268:1097-9. [PubMed]
- Grodski S, Cornford L, Sywak M, et al. Routine level VI lymph node dissection for papillary thyroid cancer: surgical technique. ANZ J Surg 2007;77:203-8. [PubMed]
- Sosa JA, Bowman HM, Tielsch JM, et al. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg 1998;228:320-30. [PubMed]
- Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet 2013;381:1058-69. [PubMed]
- Alzahrani AS, Xing M. Impact of lymph node metastases identified on central neck dissection (CND) on the recurrence of papillary thyroid cancer: potential role of BRAFV600E mutation in defining CND. Endocr Relat Cancer 2013;20:13-22. [PubMed]
- Howell GM, Nikiforova MN, Carty SE, et al. BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann Surg Oncol 2013;20:47-52. [PubMed]
- Cunningham DK, Yao KA, Turner RR, et al. Sentinel lymph node biopsy for papillary thyroid cancer: 12 years of experience at a single institution. Ann Surg Oncol 2010;17:2970-5. [PubMed]


