Primary repair and reconstruction of tumor defects in parotid masseter region: a report of 58 cases
Introduction
The anterior boundary of the parotid masseter muscle area is the anterior edge of the mandibular branch, the posterior border of the parotid masseter muscle is the superior posterior process of the sternocleidomastoid muscle and the external auditory canal, the upper boundary is the lower edge of the zygomatic arch. The common malignant tumor in the parotid gland area is parotid gland malignant tumor, skin cancer, external ear canal cancer invades parotid gland. Enlarged resection of tumors and adjacent healthy soft tissues can cause deformity of the region and even skin defects. This region is a specific region of the human body. The reconstruction involves the interdependence between aesthetics and function, should take into account defect coverage, facial contour symmetry, auricle shape and position, facial nerve protection and simultaneous restoration and reconstruction. At present, proper reconstruction of defects in this area is rare, and the strategy of reconstruction is still controversial (1). The purpose of this study is reviewed the experience of individualized reconstruction of different types of tumor defects in the parotid masseter area.
Methods
The present research was approved by the ethics committee of the Sichuan Cancer Hospital (SCCHEC2015016). The retrospective study included 58 patients from January 2014 to May 2018 with a retrospective record of cases, medical records, and pathological records (Table 1). All patients underwent extensive resection of the tumor, 35 of which underwent neck lymph node dissection.) The primary focus and flap production began at the same time to shorten the operation time and accelerate the recovery of the patients with free flap (except 2 cases of latissimus dorsi flap. Next, a group of people completed the primary lesion combined with radical resection and neck lymph node dissection. Another group of people made the myocutaneous flap. All patients underwent rapid freezing of the cutting edge and confirmed negative margin.

Full table
Results
The study lasted 48 months, including 37 (63.8%) males and 21 (36.2%) females aged 21 to 91 years. The minimum defect area was 4 cm × 5 cm, and the maximum area was 12 cm × 12 cm. Forty cases were repaired with adjacent flaps, 6 cases with major pectoralis flaps and 12 cases with free flaps. There were 7 cases of the selective facial artery, 5 cases of superior thyroid artery, 8 cases of venous choice of the facial vein, and 4 cases of superior thyroid vein.
Most of the tumors were from the parotid gland, and the pathology was mostly mucoepidermoid carcinoma (12.1%). The cutaneous squamous cell carcinoma was also a significant cause of local soft tissue defect (29.3%). A large proportion of the patients with soft tissue defects were due to local recurrence (32.8%). Male patients accounted for 63.8% of all cases. According to the size, shape, and individual condition of the patients, different repair and reconstruction schemes were selected. Most of the defects (69.1%) could be repaired by adjacent flaps, a few broad areas. The complex defect with a significant potential dead cavity can be repaired and reconstructed by pedicle or a free flap. All 58 cases of muscle flap survived. Among them, 2 cases of free flaps were relapsed after radiotherapy. The healing of the flaps and the surrounding skin was not right after the operation and healed entirely after 2 weeks of dressing change. The abdominal wall, latissimus dorsi, and lateral femoral donor area were sutured directly and recovered at the first stage. No visible secondary deformity was found, and a free skin graft was used in the forearm donor area. The patients were followed up for 6 months to 4 years. One patient with parotid carcinoma recurred locally, one with squamous cell carcinoma of parotid gland died of lung metastasis, and one with malignant melanoma died of brain metastasis. The survival rate was 56/58 (96.6%).
Typical Patient 1
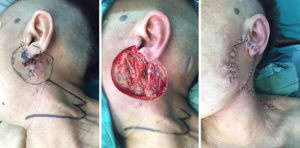
Patient, female, 61 years old, was admitted to hospital for “1 year with rupture and found a mass under the left ear lobe.” pathological biopsy of the patient’s local hospital showed basal cell carcinoma (BCC). After admission, the tumor was resected extensively, including the involved ear lobe. The incision margin was frozen negative, and the neck flap was used to repair the defect after the operation, including the primary reconstruction of the earlobe. The patient recovered well, and the appearance was satisfactory (Figure 1).
Typical Patient 2
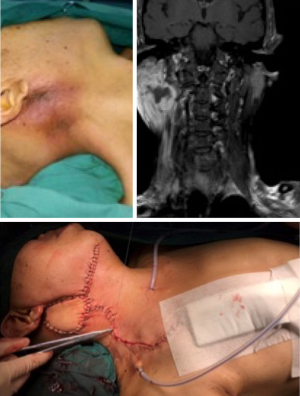
The patient was male, 55 years old, neck lymph node recurrence after radiotherapy for nasopharyngeal carcinoma, changes of local skin after radiotherapy, local skin fibrosis, due to local skin fibrosis and occlusion of small vessels after radical radiotherapy. Combined with radical resection of the tumor, there is a sizeable dead cavity, neck significant structure exposure. Therefore, the pedicled pectoralis major myocutaneous flap was selected to repair skin and subcutaneous soft tissue defects (Figure 2).
Typical Patient 3
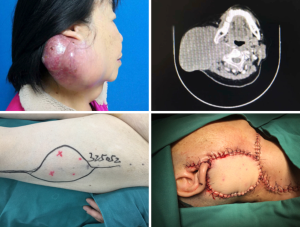
Patients, female, 74 years old, were admitted to hospital for “30 years of rapid growth and pain in the right parotid region”. The patient found the right parotid mass 30 years ago, the size of the thumb head, no tenderness, no movement, no angle of the mouth or other facial paralysis symptoms, no special treatment, six months before the rapid growth of the mass, local with burst infection. MRI findings of admission showed that the right cervical parotid region had a large space occupying change, with a range of 10.0 cm × 8.5 cm. The lesion occupied the right parotid gland, and its inner part was nodular fusion. The right jugular vein was pushed out (right internal jugular vein, part of the right external carotid artery) and the compressed lumen of the right external carotid artery and the involvement of the superior portion of the right sternocleidomastoid muscle was unclear. Push forward the right submandibular gland, close to the posterior edge of the right mandible, partly occupying and pushing the masseter muscle, occupying the subcutaneous fat space and involving the skin outwards, the adjacent skin is slightly thicker, and the upper part includes the right auricle. The right parotid tumor was resected, right neck lymph node dissected and repair and reconstruction of ALT flap after the operation (Figure 3).
Discussion
The defects in the parotid masseter region are often caused by parotid glands, primary skin tumors, or neck lymph node metastasis, invasion of adjacent soft tissues such as skin. In this study, mucoepidermoid carcinoma of the parotid gland and squamous cell carcinoma of skin are the most common. Mucoepidermoid carcinoma is easy to cause invasion of skin and masseter muscle in the advanced stage, and skin squamous cell carcinoma can also cause invasion of the parotid gland and even deep occlusal muscle. At the same time, there are more recurrent tumors in this group, and the recurrent cases are more likely to cause skin and deep soft tissue invasion. However, the defect in this area involves parotid gland and deep facial structure, and it is inevitable to leave soft tissue defects in different degrees after resection. The primary reconstruction of the region is helping to improve the quality of life of patients after operation. Because of the supporting effect of the mandible, soft tissue defects occurring in this area often cannot be sutured in situ. It is necessary to adopt individualized repair methods according to the patient’s condition and the characteristics of the defect (1,2). Many techniques can be used to repair and reconstruct the parotid gland defect after excision. The choice of the method depends on the amount of parotid gland resection and the degree of skin defect. Reconstruction should include facial contours, avoidance of Frey’s syndrome, skin coverage, tumor monitoring, and facial repair. Overall, the focus of reconstruction is to reconstruct facial contours, avoid Frey’s syndrome, provide skin coverage, minimize the harmful effects of postoperative radiotherapy, and restore facial function.
For smaller defects or parotid region cavities, the treatment of facial asymmetry after operation includes abdominal fat transplantation, injection of fillers, acellular dermal matrix (ADM), sternocleidomastoid rotation flap, local rhomboid flap. Temporal fascial rotation flap (1,3-7). In clinical work, ADM, sternocleidomastoid rotated flap was used for a long time. The complications were relatively few, and the effect was satisfactory (8,9), including latissimus cervical flap, local island flap, and so on to achieve satisfactory postoperative facial contours (10,11). In this study, it was found that the near skin flap was the main repair scheme in this area. In this group, 40 patients were repaired with the adjacent flaps because the area had a certain degree of skin activity to facilitate wound closure. The second is that the operation of the adjacent flap is relatively easy, and the time spent is less. Thirdly, the local color of the adjacent flap repair is ideal because it is the adjacent tissue. For example, in case 1 (Figure 1), soft tissue defect is designed only with a partial defect of the ear lobe, which can be repaired in one stage with satisfactory results. For the patients with large skin defect and no invasion of the deep masseter muscle, pedicle repair can be adopted according to the local and systemic conditions of the patient, covering the wound, repairing the defect and protecting the important structure of the region.
However, the complex defect with a large area or profound soft tissue defect, the adjacent flap cannot meet the needs of reconstruction. When the tumor is large, invasion of the masseter muscle, recurrence of patients after radiotherapy, patients with recurrence after postoperative radiotherapy, some patients even have a history of secondary radiation, cervical fibrosis and vascular fibrosis are vast, it is difficult to find reliable blood vessels in the recipient area when using the free flap. The neck defect after radical lymph node dissection is relatively large. In this case, the pectoralis major myocutaneous flap is a better choice. The advantage of the pectoralis major myocutaneous flap is that the position of the blood vessel is constant, the blood supply is reliable, the survival rate is high, and there is no need for vascular anastomosis. The vascular pedicled pectoralis major myocutaneous flap can not only repair the skin defect, but also cover the common carotid artery, reduce the risk of carotid artery rupture, and improve the cervical fibrosis after radiotherapy. Improve the neck shape after radical neck dissection and increase neck motion. At the same time, the tissue of pectoralis major myocutaneous flap is thicker, the blood supply is abundant, and the ability of anti-infection is stronger than that of the free flap, to facilitate the wound healing and improve the patient’s quality of life (12). Secondly, the pectoralis major myocutaneous flap is still the right choice which the hospital without microsurgical condition. In this group, six patients were repaired with the pectoralis major myocutaneous flaps. However, there are many defects of traditional the pectoralis major myocutaneous flap, especially chest malformation and loss of pectoralis primary function, could not be accepted by the patients. Therefore, our team modified the conventional pectoralis major myocutaneous flap and preserved the role and shape of pectoralis major muscle to reduce the postoperative donor sequelae (13). There are also reports on the repair of the parotid gland and lateral skull base defects by supraclavicular artery island flap, and satisfactory results have been obtained (14,15). However, the supraclavicular artery island flap is not suitable for patients with a history of cervical radiotherapy or recurrence after multiple postoperative radiotherapies.
In the advanced patients, who have experienced multiple recurrent surgeries, radiotherapy recurrence, often accompanied by the skin, masseter muscle and another invasion, some patients also accompanied by mandibular invasion, a wide range of surgery. The surface of the parotid gland area, subcutaneous muscle, and soft tissue defect may be caused after the operation, and some of them may even have the exposure of cranial base and neck vessels. For these patients, the repair of the defect after the operation is the guarantee of radical surgery. For this kind of significant defect, the local tissue flap cannot be repaired, or the facial depression is still apparent, which affects the facial contour of the patient. The repair of the major pectoralis flap sometimes appears bloated, and some patients cannot reach the upper margin of the defect. Therefore, we believe that if reliable recipient vessels can be found in the neck, the free flap should be the first choice. Twelve cases were repaired with a free tissue flap. The current free tissue flap included radial forearm flap (RFF), anterolateral thigh (ALT), deep inferior epigastric artery perforator (DIEP), lateral upper arm free (LAF) flaps and other flaps. The ALT and RFF flaps are most widely used in head and neck repair. We believe that for patients with skin cancer in the parotid region, the RFF flap can be used when the deep masseter muscle is not invaded. The flap is simple, the blood vessel is thick, the success rate is high after anastomosis, and the constant sunshine of the forearm is more similar to the color of the facial skin (1). However, when masseter muscle is invaded, the common carotid artery or internal jugular vein will often be exposed after extended resection in patients with recurrence after radiotherapy, which requires a soft tissue flap with abundant blood supply and enough tissue to repair the defect in the operation area to protect the large blood vessel.
In this case, the volume of the RFF flap is less, and the facial contour is not satisfactory. Therefore, for the significant defect of the parotid gland, the author thinks that the first choice is ALT flap, 6 cases of this group were treated with ALT flaps. We believe that the ALT flap is a multilayer composite flap which is composed of skin, adipose tissue, fascia, and lateral femoral nerve, and its blood supply comes from lateral femoral circumflex artery and vein. The ALT flap can be cut to a large area and can be made into flaps, myocutaneous flaps, multi-pedicle flaps and so on to complete the repair of various defects. The skin flap atrophy is small, the skin color is beautiful, the blood supply area is sufficient, the ability of anti-infection is strong, and its vascular diameter and superficial temporal movement: vein matching, high success rate and good tolerance to radiotherapy after the operation. The ALT flap can be used to repair the defect after total parotid gland resection. The nerve function can be reconstructed by anastomosis of the femoral nerve with the facial nerve. The lateral femoral muscle can fill the defect space after parotid gland tissue resection. The recovery of nerve function and the effect of external repair were satisfactory (16-19). The minimal donor complication is a distinct advantage of the free flap of the ALT flap. Patients are usually able to walk entirely immediately after surgery. The only long-term sequelae are linear scars. Some patients had numbness around the incision (19,20). Of course, when repairing facial skin defects, the best color match is usually a local flap, such as a facial neck flap, in which the thigh skin is paler than long-term sunburn, dark or reddish facial skin, in many people. As a result, it is difficult to obtain satisfactory facial color. It was reported that the ALT flap was combined with the cervical and facial rotated propelling flap, the epithelialized lateral thigh flap was used, and the surface was covered by the neck adjacent flap to obtain a satisfactory color match. The quality of life can be significantly improved (1,20).
In the face of the defect of parotid masseter muscle area, surgeons should carry out accurate volume reconstruction, optimize the color matching of the surrounding facial skin, and keep the anatomic position of the auricle. Kang et al. think that the free tissue transplantation of the LAF flap has a good advantage in complex parotid gland reconstruction (21). The donor site of the lateral arm of the upper arm provides functional diaphragm adipose tissue, anti-ptosis, and matches the color of the facial skin. The posterior cutaneous nerve of the upper arm can be used as the nerve graft for the rehabilitation of the facial nerve. They argue that these reconstruction advantages make the LAF flap the preferred treatment for complex parotid gland defects (21). We think that the preparation process of LAF flap is relatively easy, the flap is thin and easy to shape, and even can carry specific sensory nerve and partially restore nerve function, and the effect on the donor area is very small after the operation. However, it is necessary to recognize the thickness of the LAF flap, the greater variety of the donor vessel and caliber, the shorter pedicle, and the higher requirement of the microsurgical technique. The superior thyroid artery and the second branch end anastomosis of the external maxillary artery and the common facial vein are the best choices, and the possible end to side anastomosis is necessary (22). Also, it was reported that latissimus dorsi free flap was used to reconstruct the defect of the parotid region after the operation, and the facial nerve was reconstructed by thoracic dorsal nerve transplantation. Get an excellent cosmetic effect (23). Two cases of latissimus dorsi flap were used in this group, but we found in clinical work that patients with latissimus dorsi flap had to perform multiple postures during the operation, and the excision of the primary lesion and the manufacture of the flap could not be carried out at the same time. The operation time is relatively long, not easy for patients to recover after the operation, so we did not choose latissimus dorsi flap in a later stage.
Conclusions
We believe that the soft tissue defects in the parotid region caused by the tumor are not uncommon. The repair of the defects after the operation is the guarantee of radical surgery. Therefore, the primary repair should be completed at the same time as the radical resection of the tumor, and the facial contour should be taken into account in the repair and reconstruction. The skin covering, facial nerve function reconstruction, and facial reconstruction needs to be combined with the clinical characteristics of the defect and the individual selection of reconstruction methods of the patient’s whole body to improve the quality of life of patients after operation.
Acknowledgments
Funding: This work was supported by the Major Science and Technology Project Foundation of Sichuan Science and Technology Department (2019YFS0337), and Head and Neck Cancer Prevention and Treatment Project of Sichuan Province supported by Scientific and Technological Innovation team, China (2014TD0011).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research program was approved by the ethics committee of the Sichuan Cancer Hospital (SCCHEC2015016).
References
- Tamplen M, Knott PD, Fritz MA, et al. Controversies in Parotid Defect Reconstruction. Facial Plast Surg Clin North Am 2016;24:235-43. [Crossref] [PubMed]
- Irvine LE, Larian B, Azizzadeh B. Locoregional Parotid Reconstruction. Otolaryngol Clin North Am 2016;49:435-46. [Crossref] [PubMed]
- Pham M, Eviston TJ, Clark JR. Reconstruction of limited parotidectomy defects using the dermofat graft. ANZ J Surg 2017;87:E256-60. [Crossref] [PubMed]
- Loyo M, Gourin CG. Free abdominal fat transfer for partial and total parotidectomy defect reconstruction. Laryngoscope 2016;126:2694-8. [Crossref] [PubMed]
- Hung MH, Liao CT, Kang CJ, et al. Local Rhomboid Flap Reconstruction for Skin Defects After Excising Large Parotid Gland Tumors. J Oral Maxillofac Surg 2017;75:225.e1-5. [Crossref] [PubMed]
- Dell'Aversana Orabona G, Salzano G, Abbate V, et al. Use of the SMAS flap for reconstruction of the parotid lodge. Acta Otorhinolaryngol Ital 2015;35:406-11. [PubMed]
- Dell'aversana Orabona G, Salzano G, Petrocelli M, et al. Reconstructive techniques of the parotid region. J Craniofac Surg 2014;25:998-1002. [Crossref] [PubMed]
- Li C, Xu Y, Zhang C, et al. Modified partial superficial parotidectomy versus conventional superficial parotidectomy improves treatment of pleomorphic adenoma of the parotid gland. Am J Surg 2014;208:112-8. [Crossref] [PubMed]
- Okoturo E, Osasuyi A. Clinical Outcome of Parotidectomy with Reconstruction: Experience of a Regional Head and Neck Cancer Unit. Niger J Surg 2016;22:26-31. [Crossref] [PubMed]
- Ciocan-Pendefunda CC, Vicol C, Popescu E, et al. The platysma myocutaneous flap (PMF) for reconstruction of defects after extended parotidectomy. Rev Med Chir Soc Med Nat Iasi 2011;115:554-9. [PubMed]
- Behan FC, Lo CH, Sizeland A, et al. Keystone island flap reconstruction of parotid defects. Plast Reconstr Surg 2012;130:36e-41e. [Crossref] [PubMed]
- Chen J, Huang WX, Li Z, et al. Modified pectoralis major myocutaneous flap in reconstruction of head and neck defects. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2015;50:388-91. [PubMed]
- Cai YC, Chen J, Wang ZH, et al. Modified pectoralis major myocutaneous flap for the repair of postoperative defects of advanced parotid carcinoma Zhong Guo Zhong Liu ling Chuang Yu Kang Fu 2013;20:681-3.
- Emerick KS, Herr MW, Lin DT, et al. Supraclavicular artery island flap for reconstruction of complex parotidectomy, lateral skull base, and total auriculectomy defects. JAMA Otolaryngol Head Neck Surg 2014;140:861-6. [Crossref] [PubMed]
- Bertelsen C, Liu C, Kokot N. Reconstruction of parotidectomy and lateral skull base defects. Curr Opin Otolaryngol Head Neck Surg 2017;25:431-8. [Crossref] [PubMed]
- Deng TH, Li C, Cai YC. Advances in the application of anterolateral femoral perforator flap in the surgical application of head and neck tumors. Journal of Cancer Control and Treatment 2016;29:97-101.
- Xu ZF, Duan WY, Tan XX, et al. Reconstruction of Complex Total Parotidectomy Defect With a Chimeric Anterolateral Thigh Perforator Flap and Vascularized Motor Branch of Femoral Nerve Grafting. J Oral Maxillofac Surg 2015;73:2448.e1-7. [Crossref] [PubMed]
- Revenaugh PC, Knott PD, Scharpf J, et al. Simultaneous anterolateral thigh flap and temporalis tendon transfer to optimize facial form and function after radical parotidectomy. Arch Facial Plast Surg 2012;14:104-9. [Crossref] [PubMed]
- Fritz MA, Rolfes BN. Microvascular Reconstruction of the Parotidectomy Defect. Otolaryngol Clin North Am 2016;49:447-57. [Crossref] [PubMed]
- Ch'ng S, Ashford BG, Gao K, et al. Reconstruction of post-radical parotidectomy defects. Plast Reconstr Surg 2012;129:275e-87e. [Crossref] [PubMed]
- Kang SY, Old MO, Teknos TN. Lateral arm free tissue transfer for parotid reconstruction: A pictorial essay. Head Neck 2017;39:1015-9. [Crossref] [PubMed]
- Li C, Cai YC, Wang W, et al. The role definition of lateral arm free flap in reconstruction after head and neck cancer surgery. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2016;51:156-60. [PubMed]
- Biglioli F, Pedrazzoli M, Rabbiosi D, et al. Reconstruction of complex defects of the parotid region using a lateral thoracic wall donor site. J Craniomaxillofac Surg 2013;41:265-9. [Crossref] [PubMed]