
Nomogram for the diagnosis of suspected papillary thyroid carcinomas based on sonographic patterns: a retrospective study
Introduction
Thyroid nodules are very common and can be diagnosed in up to 67% of people (1). Ultrasonography (US) has been shown to have high sensitivity, specificity, cost-effectiveness, high availability, limited discomfort to patients (2), and an anon-ionizing nature. Up to 10–15% of thyroid nodules are confirmed to be malignant (3). Malignant nodules require timely surgical treatment, whereas patients with benign nodules can be managed conservatively, so accurate diagnosis of thyroid nodules is vital for treatment (2). However, identification of benign and malignant thyroid nodules is difficult and a consensus identification method had not been reached until now (4). Papillary thyroid carcinoma (PTC) is the most common cell type of thyroid cancer, accounting for up to 85% of all cases (5). Although, previous study has proved the role of apparent diffusion coefficient value to diagnosis of thyroid cancer (6), high resolution US is still the first choice (7). The sonographic characteristics of thyroid nodules include composition, echogenicity, margins, calcifications, shape, and vascularity (2,7). The pattern of sonographic features associated with a nodule confers malignancy risk and guides fine needle aspiration (FNA) decision making (8).
Although FNA biopsy (FNAB) is still the most reliable test for PTC detection, indeterminate cytology has been observed in up to 30% of FNABs in previous report (9). Meanwhile, this technique is invasive and associated with some complications. Thus, selective nodules suspected of being malignant should be recommended for FNAB, especially for grades 2–5 according to the Thyroid Imaging Reporting and Data System (TI-RADS) (2), in which categories 2 through 5 are divided by the possibility of malignancy. However, the scoring systems are usually too complicated for clinical application (10), meanwhile, it is discontinuous, and hardly give an accurate risk possibility, only provide an interval of malignant risk. The American Thyroid Association (ATA) guidelines provide an estimated risk of malignancy for five sonographic patterns based on the US features. However, the description of the US feature is only a text description with no digital quantification. The purpose of this study was to establish a nomogram to predict the possibility of PTC diagnosis based on the sonographic features of suspicious nodules and guide the application of FNAB.
Methods
Our retrospective study was approved by our hospital’s review board and informed consent was obtained from the patients or their families. From Aug 2016 to Dec 2017, Clinical and US data from PTC patients were collected and evaluated at Gansu Provincial Hospital. Five hundred and thirty-two patients were excluded from our study, including 12 due to follicular or medullary thyroid carcinoma, 26 for inadequate sonographic data acquisition, 11 for history of thyroid surgery, 22 for multiple suspicious nodules, and 461 patients for multiple thyroid nodules. A total of 382 patients with solitary nodules were included. All these patients accepted a thyroidectomy, and the diagnosis was confirmed by postoperative histological pathologic examination. According to the pathological examination, the 382 patients were divided into two groups: the PTC group and the benign nodule group. Near-total or total thyroidectomy as well as lobectomy were performed in all cases. The final diagnosis of the nodules was determined by pathological examination. The baseline characteristics and sonographic features of the two groups were compared.
All patients underwent a thyroid nodule US examination 3 months before surgery. Sonographic features of thyroid nodules were evaluated and scored for composition (solid =2, cystic proportion =1, or spongiform =0), echogenicity (hypoechoic =2, isoechoic=1 or hyperechoic =0), margins (irregular =1 or regular =0), calcifications (microcalcifications with calcifications smaller than 1.0 mm =2, no calcifications =1, macrocalcifications =0), shape (taller than wide =1, no =0), and internal vascularization (high =2, medium =1, low or none =0) according to the ATA guidelines (2). Other characteristics that were recorded and included in our analysis included border type (obscure =1, clear =0), posterior echo (attenuation =1, no attenuation =0), and halo (present =0, absent =1) (11), as well as nodule size (in three dimensions) and location (e.g., left upper lobe). Sonographic examination and evaluation were carried out by two independent sonographic radiologists who were blinded to the pathological outcomes using standard procedures. Both of these observers had at least 5 years of experience in thyroid sonography. In case of disagreement, the two experts worked collaboratively to form a consensus. All sonographic examinations were performed with the patients in a supine position using a color Doppler Philip iE33 ultrasound machine (GELogiq E9, USA) and 6–8 or 10–12 MHz linear probe (ML6-15-D or 9L-D) according to thyroid nodule location. Fine needle aspiration cytology (FNAC) was performed only for patients with suspicious US features. Through a single needle puncture and multiple needle motions under local anesthesia, samples within needles were fixed in 95% ethanol and examined after Papanicolaou staining.
The risk factors for PTC reported in other studies were analyzed in our patients as a primary cohort to develop the nomogram for predicting PTC diagnosis. Then, based on the same selection criteria, we prospectively collected another independent cohort of 162 patients in our hospital that served as the validation cohort.
Continuous variables were expressed as the means ± standard deviation and compared between the two groups using the t-test, and categorical variables were expressed as rates and compared using the chi-square test. Univariate analysis was used to assess the relationship between sonographic parameters and histological diagnosis. Statistically significant variables (P<0.05) were further analyzed by multiple logistic regression analysis. Univariate and multivariate analyses were performed using the Cox proportional hazards model. The nomogram was built based on the results of multivariate analyses. The final model selection for the nomogram was performed by a backward step-down selection process using the Akaike information criterion (12). Nomograms were generated using the rms package of R software. All other statistical tests were performed with SPSS software (Version 17.0, Chicago, IL, USA). A P value less than 0.05 was considered significant.
Results
As shown in Table 1, the primary and validation cohorts included 382 and 162 patients, respectively. Of the 544 included patients, 415 (76.3%) patients had PTC. The baseline clinical and tumor characteristics were broadly similar between the two groups. Meanwhile, preoperative US features were also comparable between the two groups in terms of composition, echogenicity, margins, calcifications, shape, internal vascularization, border, posterior echo, and halo. So, the primary and validation cohorts are comparable.
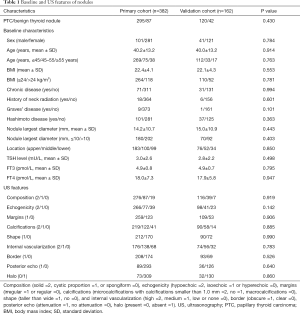
Full table
Based on baseline and US features, PTC patients and those with benign nodules in the primary cohort were compared by univariate analysis. As shown in Table 2, although there were gender differences in patients with Hashimoto disease and tumor diameters between the two groups, these differences did not reach statistical significance (P>0.05). According to the US features, PTC patients showed more lesions that were solid, hypoechoic, taller than wide in shape, and with irregular margins, microcalcifications, high internal vascularization, and obscure borders (P<0.002 for all characteristics). However, posterior echo and halo did not show any differences between PTC and benign nodule patients (P≥0.100).
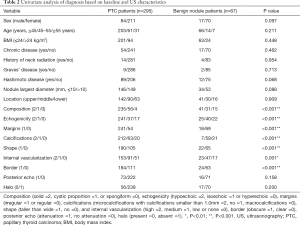
Full table
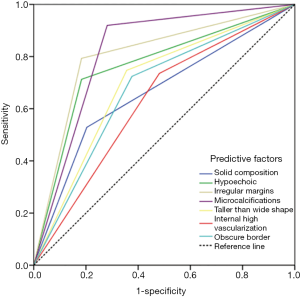
Multivariate analyses included all variables that are considered significant in the univariate analysis (P<0.05), and each of these seven factors were divided into binary variables. As shown in Table 3, the results indicated that all seven factors of US features were the risk factors for PTC and can contribute to the diagnosis of PTC compared to benign nodule US features (all P values were less than 0.01). Receiver operating characteristic (ROC) curve analysis were performed and compared among these seven US features, as showed in Figure 1, microcalcification has the largest area under ROC curve, and internal high vascularization has the smallest.

Full table
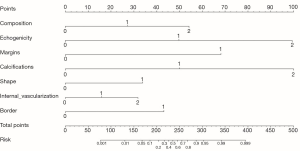
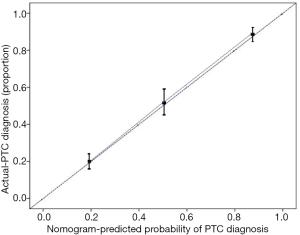
Based on the univariate and multivariate analyses of PTC diagnosis, the seven risk factors based on the US features were incorporated into the PTC diagnosis nomogram, as shown in Figure 2. An individual nodule’s value is loaded on each variable axis (the 2nd–8th lines) and a line is drawn upwards to determine the number of points received for each variable value (the first line); the sum of these numbers is located on the total point axis (9th line), and a line is drawn downwards to the risk axes (10th line) to determine the likelihood of PTC diagnosis (total point = Composition’ score + Echogenicity’ + Margins’ + Calcifications’ + Shape’ + Internal Vascularization’ +Border’, and likelihood of PTC diagnosis can be got according to the total point). In the validation cohort, the baseline characteristics and US features were comparable to the primary cohort. The C-index of the nomogram for predicting PTC diagnosis based on the US features was 0.87 (95% CI: 0.81–0.92), and a calibration curve showed good agreement between prediction and observation (Figure 3). These results indicate a high predictive ability of this nomogram.
Discussion
Ultrasonographic-guided FNAB is used to diagnose suspicious nodules for thyroid cancer (2). However, there are some disadvantages, such as inadequate sampling, high cost, invasiveness, indeterminate cytology in some cases, and operator dependency (13,14). Thus, accurate PTC diagnosis using only US is very valuable. The goal of our present study was to establish a nomogram based on US features to precisely diagnose PTC nodules.
The US features for PTCs included in our present study were based on the ATA guidelines (2) and other previous related studies (1,11,14). Previous studies have shown that several US features, such as nodular margins, blood flow distribution, hypoechoic appearance, and microcalcification are associated with PTC malignancy, which was consistent with our multivariate analysis (15,16). We included nine characteristics of US in our research, and, to the best of our knowledge, this is the first study with so many US features for diagnosis of PTC (17,18). However, we did not include sonographic elastography, which is analyzed with related software and probes compared to US (19). The main reason was use of this technique in thyroid nodules applied to only a few patients in our center due to the lack of software.
In our analysis, we included 25 factors that are reported to possibly be related to the risk of PTC (14,20). However, our result detected only US features that can be used to distinguish benign nodules and PTCs. The earliest research on the US features to distinguish benign and malignant thyroid nodes was published by Horvath et al. (21). They described ten sonographic patterns of thyroid nodules and the associated risk of malignancy. Similar studies were subsequently published (22,23); however, it is too difficult to classify each nodule with the entire complex equation. Meanwhile, the categorizing method was too complicated for clinical use, and the suspicious features were assigned the same weight for predicting the nature of a nodule (2). We established a simple, visual, numeric version of a predictive system based on the US features, which is very important for clinical usage. Xu and colleagues (11) established a scoring and categorizing method based on sonographic features. However, the scoring system was built based on the odds ratio of multivariate analysis. Risk factor scores were simply added; thus, a precise visual system was not developed in this paper. To the best of our knowledge, this is the first study to establish a nomogram based on US features to diagnose PTCs based on the risk factors of multivariate analysis. Each risk factor is assigned a score, which are summed and represented in the nomogram. The nomogram has been used for various human cancers, such as liver (24), laryngeal (25), lung (26), pancreatic (27), colon (28), and breast cancer (29). The prospective cohort in our hospital was used to validate the effectiveness of this nomogram and obtain a high C-index. We report that the nomogram based on US features was effective to diagnose PTC.
Our study does have some limitations. Firstly, there was unavoidable selection bias due to the lack of histological data in ultrasonically small and benign thyroid nodules that have no indication for FNA or surgery. However, long-term follow-up may also demonstrate the benign characteristics. Secondly, the limited patient numbers in a single center may temper our results and conclusions. However, the nomogram was not only built but also validated by a prospective cohort. Furthermore, a multiple center, large cohort study will be performed to further validate the nomogram. Finally, the ultrasonic elastography did not include our present study due to the lack of these data, however, from now on, the ultrasonic elastography will be recommended to suspicious patient, and in our future publication, these data will be present.
In conclusion, we developed a nomogram that could accurately and objectively diagnose PTC nodules based on US features.
Acknowledgments
Funding: This study was supported by grant from Gansu Provincial Hospital Project (17GSSY6-11,2019-166).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our retrospective study was approved by our hospital’s review board (No. 2019-125) and informed consent was obtained from the patients or their families.
References
- Guth S, Theune U, Aberle J, et al. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest 2009;39:699-706. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Eszlinger M, Lau L, Ghaznavi S, et al. Molecular profiling of thyroid nodule fine-needle aspiration cytology. Nat Rev Endocrinol 2017;13:415-24. [Crossref] [PubMed]
- Ye L, Zhou X, Huang F, et al. The genetic landscape of benign thyroid nodules revealed by whole exome and transcriptome sequencing. Nat Commun 2017;8:15533. [Crossref] [PubMed]
- Zimmermann MB. Thyroid gland: Iodine deficiency and thyroid nodules. Nat Rev Endocrinol 2014;10:707-8. [Crossref] [PubMed]
- Razek AA, Sadek AG, Kombar OR, et al. Role of apparent diffusion coefficient values in differentiation between malignant and benign solitary thyroid nodules. AJNR Am J Neuroradiol 2008;29:563-8. [Crossref] [PubMed]
- Li X, Zhang S, Zhang Q, et al. Diagnosis of thyroid cancer using deep convolutional neural network models applied to sonographic images: a retrospective, multicohort, diagnostic study. Lancet Oncol 2019;20:193-201. [Crossref] [PubMed]
- Colombo C, Verga U, Mian C, et al. Comparison of calcium and pentagastrin tests for the diagnosis and follow-up of medullary thyroid cancer. J Clin Endocrinol Metab 2012;97:905-13. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol 2009;132:658-65. [Crossref] [PubMed]
- Russ G, Bigorgne C, Royer B, et al. The Thyroid Imaging Reporting and Data System (TIRADS) for ultrasound of the thyroid. J Radiol 2011;92:701-13. [Crossref] [PubMed]
- Xu SY, Zhan WW, Wang WH. Evaluation of Thyroid Nodules by a Scoring and Categorizing Method Based on Sonographic Features. J Ultrasound Med 2015;34:2179-85. [Crossref] [PubMed]
- Li J, Zhou J, Yang PH, et al. Nomograms for survival prediction in patients undergoing liver resection for hepatitis B virus related early stage hepatocellular carcinoma. Eur J Cancer 2016;62:86-95. [Crossref] [PubMed]
- Kim EK, Park CS, Chung WY, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol 2002;178:687-91. [Crossref] [PubMed]
- Unsal O, Akpinar M, Turk B, et al. Sonographic scoring of solid thyroid nodules: effects of nodule size and suspicious cervical lymph node. Braz J Otorhinolaryngol 2017;83:73-9. [Crossref] [PubMed]
- Moon WJ, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology 2008;247:762-70. [Crossref] [PubMed]
- Tamsel S, Demirpolat G, Erdogan M, et al. Power Doppler US patterns of vascularity and spectral Doppler US parameters in predicting malignancy in thyroid nodules. Clin Radiol 2007;62:245-51. [Crossref] [PubMed]
- Shin JH. Ultrasonographic imaging of papillary thyroid carcinoma variants. Ultrasonography 2017;36:103-10. [Crossref] [PubMed]
- Wei X, Li Y, Zhang S, et al. Meta-analysis of thyroid imaging reporting and data system in the ultrasonographic diagnosis of 10,437 thyroid nodules. Head Neck 2016;38:309-15. [Crossref] [PubMed]
- Menzilcioglu MS, Duymus M, Avcu S. Sonographic Elastography of the Thyroid Gland. Pol J Radiol 2016;81:152-6. [Crossref] [PubMed]
- Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab 2013;98:564-70. [Crossref] [PubMed]
- Horvath E, Majlis S, Rossi R, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab 2009;94:1748-51. [Crossref] [PubMed]
- Park JY, Lee HJ, Jang HW, et al. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid 2009;19:1257-64. [Crossref] [PubMed]
- Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 2011;260:892-9. [Crossref] [PubMed]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- Multidisciplinary Larynx Cancer Working Group. Conditional Survival Analysis of Patients With Locally Advanced Laryngeal Cancer: Construction of a Dynamic Risk Model and Clinical Nomogram. Sci Rep 2017;7:43928. [Crossref] [PubMed]
- Zhang B, Yuan Z, Zhao L, et al. Nomograms for predicting progression and efficacy of post-operation radiotherapy in IIIA-pN2 non-small cell lung cancer patients. Oncotarget 2017;8:37208-16. [PubMed]
- Shimizu Y, Yamaue H, Maguchi H, et al. Validation of a nomogram for predicting the probability of carcinoma in patients with intraductal papillary mucinous neoplasm in 180 pancreatic resection patients at 3 high-volume centers. Pancreas 2015;44:459-64. [PubMed]
- Frasson M, Flor-Lorente B, Rodriguez JL, et al. Risk Factors for Anastomotic Leak After Colon Resection for Cancer: Multivariate Analysis and Nomogram From a Multicentric, Prospective, National Study With 3193 Patients. Ann Surg 2015;262:321-30. [Crossref] [PubMed]
- Zhang J, Li X, Huang R, et al. A nomogram to predict the probability of axillary lymph node metastasis in female patients with breast cancer in China: A nationwide, multicenter, 10-year epidemiological study. Oncotarget 2017;8:35311-25. [PubMed]


