
Neuroblastoma image-defined risk factors in adrenal neuroblastoma: role of radiologist
Introduction
Neuroblastoma (NBL) is the most common extracranial solid malignancy in children. Neuroblastoma originates from the sympatico-adrenal lineage of the neural crest and can grows anywhere along the sympathetic nervous system chain (1). The incidence is more than 7% in childhood each year and the median age of diagnosis is 15 months (2). The tumor develops in the abdomen, in particular localized in the adrenal glands (49%). Other sites of primary lesions include: neck (1%), chest (19%), abdomen (30%, non-adrenal), or pelvis (1%) (3). Hepatic, lymphatic, bone marrow and bone cortical infiltration metastasis disease are present at diagnosis in 50% of patients (1). The etiology of Neuroblastoma is unknown. The main cases are sporadic, others are described in literature in association with Neurofibromatosis type I, Beckwith-Weidemann syndrome, Hirschsprung’s disease and DiGeorge syndrome and almost 1% are familiar, presenting an autosomal dominant (AD) pattern with incomplete penetrance. Recent studies considered the association between consumption of substances including tobacco, alcohol or marijuana during pregnancy and risk of neuroblastoma (4).
Patient age, tumor stage, amplification of MYCN (20%) and ALK oncogene and DNA ploidy, specific chromosomal alterations include gain of 17q and deletion of 11q and 1p36 and grade of tumor differentiation are involved in tumor prognosis (1,3,5-7). There are some typical microscope characteristics of Neuroblastoma (small, round, blue cells clustered in rosettes) in common with other frequent tumors in childhood like Ewing’s sarcoma, primitive neuroectodermal tumors (PNETs), leukemia, lymphoma and rhabdomyosarcoma (1,8).
Symptoms are variable and depend on site of primary tumors. Usually, in symptomatic patients with localized tumors, an incidentally abdominal mass or abdominal distension is the typical evidence, sometimes associated with abdominal pain. Other symptoms include Horner’s syndrome (thoracic localization), hypertension (as a result of the compression of renal vessels) and neurologic deficits. However, patients with localized disease are often asymptomatic. Also paraneoplastic syndromes may be present in localized disease, the most common syndromes are opsomyoclonus (consists in rapid eye movements, ataxia, and irregular muscle movements) and Kerner-Morrison syndrome (consists in hypersecretion of vasoactive intestinal peptide (VIP) resulting in watery diarrhea) (1,2,9). Sometime the diagnosis is secondary to systematic symptoms like fever or weight loss; in patients with flushing, tachycardia or hypertension it is important to dose urinary catecholamine (10).
Neuroblastoma has highly variable biologic behavior: in fact tumors may spontaneously regress, differentiate into benign ganglioneuromas or follow an unrelenting progressive course. High-risk features, including large, unresectable tumors and widely metastatic disease, are present in up to 50% of patients. Long-term survival of these patients is about 40% (1,8).
Imaging
The diagnosis of neuroblastoma is suggested on imaging pattern and age of the patient, and the most challenge is to differentiate neuroblastoma, retroperitoneal extrarenal tumor, from Wilms tumor, which is retroperitoneal renal tumor, also found in the same age range.
The imaging characteristics of neuroblastoma, ganglioneuroblastoma, and ganglioneuroma are similar, and for this reason the histologic type cannot be discriminated with imaging but is an exclusively anatomopathological diagnosis (3).
The diagnostic approach in childhood is based on ultrasound (US), particularly for abdominal mass.
US allows the detection of the primary lesion, it can also identify the relationship with neighboring structures (organs/vessels) and describe some characteristics because it is well tolerated in children, easy to find, low cost and doesn’t use radiation (11). The first evidence is a heterogeneous vascularized solid mass, which is associated with frequent presence of contextual calcifications or, more rarely, of the cystic component (11,12) (Figure 1A).
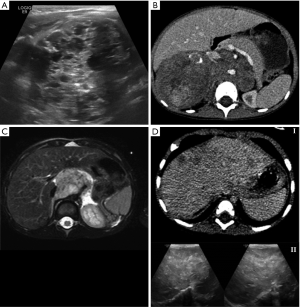
In addition to this, it is useful to perform a color Doppler examination, to discriminate between vascular compression and vascular invasion. There’s also an increase in diagnoses of fetal or congenital neuroblastoma due to a large diffusion of this modality in neonatal screening. This variable entity must be differentiated from fetal adrenal hemorrhage: the latter appears as a maturing mass during follow-up US and doesn’t show any sign on Doppler US (11).
There’s also an increase in the diagnoses of fetal or congenital neuroblastoma due to a large diffusion of this modality in neonatal screening. This variable entity must be differentiated from fetal adrenal hemorrhage: the latter appears as a maturing mass during follow-up US and doesn’t show sign on Doppler US (12).
Inter-observer reproducibility and acoustic shadowing caused by calcifications limit the use of ultrasound examination.
X-ray, performed for other reasons, may incidentally show the presence of a mass that compresses adjacent structures and presents contextual calcifications. It can also demonstrate lytic lesions or metaphyseal lucencies in metastatic disease, but it has low sensibility and specificity (2).
To define the disease correctly and for treatment planning multiplanar imaging modalities are necessary at the time of diagnosis, and for this scope magnetic resonance (MR) or computed tomography (CT) can be used.
In accordance with literature, for the assessment of the local disease there’s no agreement about the use of CT or MR.
CT is generally performed for planning surgical treatment, especially for the most confident of surgeons with this imaging modality.
On CT, neuroblastoma often appears as a heterogeneous mass, with indistinct margins, calcifications which are present in 80–90% (13), areas of necrosis, hemorrhage and contrast enhancement. The lesion can also cross the midline and involve other anatomic compartments, and generally encase and displace structures rather than invade them (1).
The use of multiplanar reconstructions and maximum intensity projection (MIP) allows vascular evaluation for staging tumor, for a best definition of the primary lesion and for the treatment (Figure 1B).
CT is performed using single spiral acquisition, generally at 40–60 s after contrast media administration, to reduce the radiation dose.
It’s great availability, high-quality lesion detection and the ability to perform scan without patient sedation are the strengths of this modality (14). It places limitations on the radiant dose in more susceptible basic patients (15,16) and the use of contrast media, used to improve tumor detection and its relation with neighboring structures, as vessels.
Nowadays the dual-source CT allow the evaluation of both contrast enhancement and calcification with one acquisition, thanks to software reconstruction, which results in reduction of both radiant dose and contrast media (17). With the new CT scan the acquisition time is very fast, reduces movement artifacts, so sedation is not required (1,3).
CT is also is required to plan the radiation field and the dose for radiation therapy (18), but nowadays there’s an increased use of the MR to detect the target lesion (19).
After chemotherapic treatment, CT better evaluates than MR solid portion of the mass and calcification, increased because related to necrosis, that it important before surgical approach.
MR, when available, is the mainstay modality for the evaluation of primary tumor in all different compartments.
The mass appears heterogeneously hypointense in T1 and hyperintense in T2, sometimes with cystic aspects and contextual hemorrhagic areas (Figure 1C). Contrast enhancement is variable, and diffusion on diffusion-weight imaging (DWI) may be restricted in more malignant forms for the high cellularity (20). One advantage, when compared to CT, is the possibility to evaluate vessels, and thus the local-regional tumor extent, without the use of contrast media, also considering the recent limitations on gadolinium-based contrast agents due to their accumulation (21). Some authors believe that the use of diffusion weight pulse sequences, in addition to best definition of soft tissues, allow best evaluation of staging and IDRFs with MR (22).
Limits are represented by the low availability, poor assessment of calcifications and the sedation of the patient to prevent movement artifacts.
MR is more sensitive than CT to identify bone marrow involvement, invasion of the spinal cord and for liver metastasis, especially in the diffusely infiltrative forms (1).
Whole-body MR is now a modality to evaluate metastatic disease to bone marrow, using combinations of T1 weighted sequences after and before contrast injection, but its role is still unclear (23,24). With Whole-body MR signal intensity abnormalities have low specificity when compared to I-123 metaiodobenzylguanidine (I-123 MIBG), and there isn’t a standardization or score for the evaluation of the disease. Whole- body MR is not accurate to detect extraskeletal sites of metastatic disease.
Nuclear medicine is useful in detection of occult disease and for bone marrow (70%) and bone (55%) metastasis, that are the most frequently involved sites in metastatic disease and confirmed by bone marrow bilateral aspirate and biopsy.
I-123 MIBG can detect MIBG-avid disease (which expresses norepinephrine transporter) with a high sensitivity (90%) and specificity (nearly 100%) (25). Limits are represented by low spatial resolution, particularly of planar imaging, and by the fact that some tumors don’t have a norepinephrine transporter (a small percentage of neuroblastoma). Complementary investigations, useful for staging, are the bone scintigraphy, for the evaluation of bone metastases, and PET-CT.
Technetium Tc-99m medronate bone scintigraphy isn’t used routinely, but it’s only performed when the tumor is not MIBG avid or MIBG positivity cannot be confirmed (26). One of the most important limit of the Tc-99m scintigraphy is the physiological uptake of the growing metaphysis in children, which can be wrongly interpreted as metastasis (27).
The role of 18-F fluorodeoxyglucose positron emission tomography (18-F FDG PET) is still unclear (28). It can be helpful in detecting small lesions and in tumors that are MIBG-negative (10%), and can play a role for initial disease staging at diagnosis, to monitor response during treatment and for follow-up. It must be considered that 18-F FDG PET has low specificity, due to uptake also in inflammatory conditions. For this reason it must be used in selected cases, as when there is a discrepancy between MIBG and CT/MR (29).
Staging
The staging system for prognostic purpose was one of the first points of disagreement, which led to the International Neuroblastoma Staging System (INSS) of 1986, revised in 1989, which relies on surgical staging (30). This staging system describes the characteristics of the primary lesion, the loco-regional lymph node involvement and invasion of the neighboring organs, and the presence of metastasis. INSS was made on imaging before and after surgery: the main problem was the lack of accordance between surgical and imaging staging (31).
Furthermore, INSS doesn’t evaluate that some tumors are not operated because in the case of localized disease spontaneous regression can occur.
There’s also a difficulty to evaluate uniformly lymph node interested by the disease.
The limit of this classification was the different surgical resection, also done at interval times from diagnosis. To overcome this difficulty a new staging system was made based on preoperative imaging by the International Neuroblastoma Risk Group (INRG) in 2009 (32). This new staging system uses 20 Image-Defined Risk Factors (IDRFs) across multiple organ systems, and must be considered in parallel to the INSS staging system. The aim of this IDRFs is to predict surgical outcomes and, in addition with clinical data, to provide risk stratification. The INRG Staging System (INRGSS) relies on Imaging-Defined Risk Factors (IDRFs) that are determined before surgery or other therapy (33).
With the application of the INRGSS the radiologist’s role in staging children with neuroblastoma increased.
According to this classification, localized disease is classified as L1 or L2, while metastatic disease as stage M or MS.
Stage L1 is used for a tumor that doesn’t involve vital structures, according with IDRFs, and limited to one body compartment.
Stage L2 indicates lesion with at least one IDRF; the lesion can involve two compartments, but must be ipsilateral (for example a disease extended in the abdomen and chest on the left side).
Stage M is for metastatic disease (not contiguous with the primary lesion), and it includes also the involvement of nonregional lymph node. If there’s an involvement of mediastinal lymph nodes in a case of tumor localized in the upper abdomen, or involvement of the inguinal lymph nodes in pelvic mass, it is not considered metastatic disease but locoregional.
Ascites and pleural effusion, even with malignant cells, are not considered metastatic disease unless they are distant from the body compartment of the primary tumor.
Stage MS refers to metastatic disease to skin, liver and/ or bone marrow in children younger than 18 months (Figure 1D). Bone marrow involvement should be limited to less than 10% of all nucleated cells in the culture smears or biopsy samples. I123-MIBG scintigraphy findings must be negative in bone and bone marrow (3).
Imaging characteristics of IDRFs
The use of INRGSS requires a deep knowledge of the terms used to describe the IDRFs correctly.
It’s very important that every radiologist uses the same language, to allow better communication between radiologists themselves and with the surgeons.
IDRFs are also useful for the follow up, both for patients who are observed only or treated with primary chemotherapy, to document tumor regression or as a preoperative assessment. When imaging is repeated in follow-up protocol, reporting should include reassessment of the patient’s IDRF status.
IDRFs used for the evaluation of neuroblastoma that involves the adrenal gland are (3):
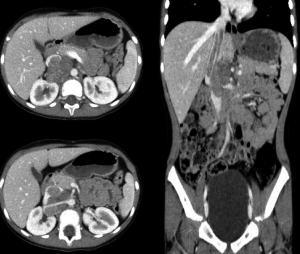
- Contact: there’s no visible layer between tumor and normal tissue and/or there is involvement of less than 50% of the vessel’s circumference (Figure 2). The contact with the renal artery, vein or renal pelvis is considered an invasion of the kidney (Figure 3);
- Encasement: there isn’t a visible layer of normal tissue between tumor and neighboring vessel. There’s an involvement of more than 50% of the circumference of the artery, whereas the veins appear flattened, without visible lumen (Figure 4);
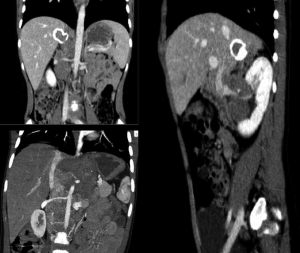
- Infiltration: malignant tissue grows into organ with loss/ill-defined margins (Figure 5);
- Invasion: there’s a contact or encasement of the renal vessels (Figure 6).
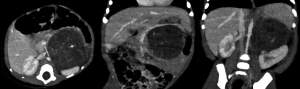
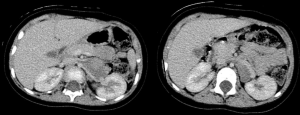
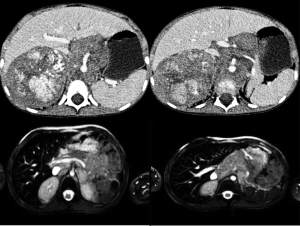
Separation (when there’s a visible layer of normal tissue between tumor and other structures) and flattened (when the tumor compresses veins, which maintain a partially visible lumen) they are not considered IDRFs, but they have to be known for correctly reporting.
The leading point is establish relationship between local disease and main abdominal vessels prior to performing surgery, and the challenge for the radiologist is to do an accurate and complete report. IDRFs are positive when there is:
- infiltration of porta hepatis and/or hepatoduodenal ligament;
- encasing of branches of superior mesenteric artery at mesenteric root;
- encasing of the origin of the celiac axis and/or of the superior mesenteric arteries;
- invasion of one or both renal pedicles;
- encasing of the aorta and/or vena cava (Figure 7);
- encasing of the iliac vessels.
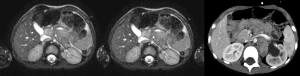
An encasement of the inferior mesenteric artery is not considered as an IDRF, because if damaged during surgery, it doesn’t lead to complications.
Another IDRF is the infiltration of adjacent organs and structures, such as pericardium, diaphragm, kidney, liver or duodeno-pancreatic block.
A tumor extension through neural foramina and occupies more than one third of the spinal canal in the axial plane above the level of vertebral body of L2, with disappearance of the perimedullary leptomeningeal spaces or with abnormal spinal cord signal intensity is considered an IDRF. When the mass develops in the spinal cord below the vertebral body of L2, it is not an IDRF.
The majority of neuroblastoma develop in one anatomic compartment, but they can extend into an adjacent compartment: in the latter case must be considered an IDRF (abdomen-chest; abdomen-pelvis). There’s the possibility of a multifocal primary neuroblastoma, often familial, which can be metachronous or synchronous: in this case, each neoplasm should be staged according to the greatest extent of disease, but for itself is not considered an IDRF.
Differential diagnoses
The main differential diagnoses is with Nephroblastoma/Wilms’ tumor.
Wilms’ tumor rarely presents calcification (<10%), often with a curvilinear pattern.
Nephroblastoma occasionally involves para-aortic lymph nodes, but often invades the inferior vena cava. Metastases are common to lungs.
Conclusions
In conclusion, INRGSS is the gold standard for preoperative staging of neuroblastoma, because it allows reproducibility, essential to compare results in clinical trials, and improves communication with surgeons and other clinicians.
Imaging role is decisive both in diagnosis, staging and for treatment planning, because it delineates IDRFs: this definitively changes the approach to this pathology.
CT and/or MR are both used to characterize primary tumor, metastatic disease and evaluate IDRFs in INRGSS, but MR is the choice modality in follow-up for reducing radiant dose exposition.
MIBG scintigraphy is an important tool to define the metastatic disease, but the role of MR is increasing.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Dumba M, Jaward N, McHung K. Neuroblastoma and nephroblastoma: a radiological review. Cancer Imaging 2015;15:5. [Crossref] [PubMed]
- Liu W, Zheng J, Li Q. Application of imaging modalities for evaluating neuroblastoma. J Pediatr Endocrinol Metab 2013;26:1015-20. [Crossref] [PubMed]
- Brisse HJ, McCarville MB, Granata C, et al. International Neuroblastoma Risk Group Project. Guidelines for imaging and staging of neuroblastic tumors: consensus report from the International Neuroblastoma Risk Group Project. Radiology 2011;261:243-57. [Crossref] [PubMed]
- Müller-Schulte E, Kurlemann G, Harder A. Tobacco, alcohol and illicit drugs during pregnancy and risk of neuroblastoma: systematic review. Arch Dis Child Fetal Neonatal Ed 2018;103:F467-73. [Crossref] [PubMed]
- Fletcher JI, Ziegler DS, Trahair TN, et al. Too many targets, not enough patients: rethinking neuroblastoma clinical trials Nat Rev Cancer 2018;18:389-400. [Crossref] [PubMed]
- Sharma R, Mer J, Lion A, et al. Clinical Presentation, Evaluation, and Management of Neuroblastoma. Pediatr Rev 2018;39:194-203. [Crossref] [PubMed]
- Swift CC, Eklund MJ, Kraveka JM, et al. Updates in Diagnosis, Management, and Treatment of Neuroblastoma Radiographics 2018;38:566-80. [Crossref] [PubMed]
- McCarville MB. Imaging neuroblastoma: what the radiologist needs to know. Cancer Imaging 2011;11 Spec No A:S44-7.
- Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet 2007;369:2106-20. [Crossref] [PubMed]
- Tolbert VP, Matthay KK. Neuroblastoma: clinical and biological approach to risk stratification and treatment. Springer-Verlag GmbH Germany, part of Springer Nature, 2018.
- David R, Lamki N, Fan S, et al. The many faces of neuroblastoma. Radiographics 1989;9:859-82. [Crossref] [PubMed]
- Deeg KH, Bettendorf U, Hofmann V. Differential diagnosis of neonatal adrenal haemorrhage and congenital neuroblastoma by colour coded Doppler sonography and power Doppler sonography. Eur J Pediatr 1998;157:294-7. [Crossref] [PubMed]
- Mehta K, Haller JO, Legasto AC. Imaging neuroblastoma in children. Crit Rev Comput Tomogr 2003;44:47-61. [Crossref] [PubMed]
- Callahan MJ, MacDougall RD, Bixby SD, et al. Ionizing radiation from computed tomography versus anesthesia for magnetic resonance imaging in infants and children: patient safety considerations. Pediatr Radiol 2018;48:21-30. [Crossref] [PubMed]
- Xu Y, Wang J, Peng Y, Zeng J. CT characteristics of primary retroperitoneal neoplasms in children. Eur J Radiol 2010;75:321-8. [Crossref] [PubMed]
- Brody AS, Frush DP, Huda W, et al. American Academy of Pediatrics Section on Radiology. Radiation risk to children from computed tomography. Pediatrics 2007;120:677-82. [Crossref] [PubMed]
- Martine RJ, Santangelo T, Colas L, et al. Radiation dose levels in pediatric chest CT: experience in 499 children evaluated with dual-source single-energy CT. Pediatr Radiol 2017;47:161-8. [Crossref] [PubMed]
- Gatcombe HG, Marcus RB, Katzenstein HM, et al. Excellent local control from radiation therapy for high-risk neuroblastoma. Int J Radiat Oncol Biol Phys 2009;74:1549-54. [Crossref] [PubMed]
- Jonsson JH, Karlsson MG, Karlsson M, et al. Treatment planning using MRI data: an analysis of the dose calculation accuracy for different treatment regions. Radiat Oncol 2010;5:62. [Crossref] [PubMed]
- Sofka CM, Semelka RC, Kelekis NL, et al. Magnetic resonance imaging of neuroblastoma using current techniques. Magn Reson Imaging 1999;17:193-8. [Crossref] [PubMed]
- Ranga A, Agarwal Y, Garg KJ. Gadolinium based contrast agents in current practice: Risks of accumulation and toxicity in patients with normal renal function. Indian J Radiol Imaging 2017;27:141-7. [Crossref] [PubMed]
- Uhl M, Altehoefer C, Kontny U, et al. MRI-diffusion imaging of neuroblastomas: first results and correlation to histology. Eur Radiol 2002;12:2335-8. [Crossref] [PubMed]
- Mazumdar A, Siegel MJ, Narra V, et al. Whole-body fast inversion recovery MR imaging of small cell neoplasms inpediatric patients: a pilot study. AJR Am J Roentgenol 2002;179:1261-6. [Crossref] [PubMed]
- Kellenberger CJ, Epelman M, Miller SF, et al. Fast STIR whole-body MR imaging in children. Radiographics 2004;24:1317-30. [Crossref] [PubMed]
- Matthay KK, Shulkin B, Ladenstein R, et al. Criteria for evaluation of disease extent by 123I-metaiodobenzylguanidine scans in neuroblastoma: a report for the InternationalNeuroblastoma Risk Group (INRG) TaskForce. Br J Cancer 2010;102:1319-26. [Crossref] [PubMed]
- Sharp SE, Trout AT, Weiss BD, et al. MIBG in neuroblastoma diagnostic imaging and therapy. Radiographics 2016;36:258-78. [Crossref] [PubMed]
- Jacobs A, Delree M, Desprechins B, et al. Consolidating the role of I-MIBG-scintigraphy in childhood neuroblastoma: five years of clinical experience. Pediatr Radiol 1990;20:157-9. [Crossref] [PubMed]
- Sharp SE, Shulkin BL, Gelfand MJ, et al. 123I-MIBG scintigraphy and 18F-FDG PET in neuroblastoma. J Nucl Med 2009;50:1237-43. [Crossref] [PubMed]
- Papathanasiou ND, Gaze MN, Sullivan K, et al. 18F-FDG PET/CT and 123I-metaiodobenzylguanidine imaging in high-riskneuroblastoma: diagnostic comparison and survival analysis. J Nucl Med 2011;52:519-25. [Crossref] [PubMed]
- Brodeur GM, Seeger RC, Barrett A, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol 1988;6:1874-81. [Crossref] [PubMed]
- Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993;11:1466-77. [Crossref] [PubMed]
- Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group(INRG) staging system: an INRG Task Force report. J Clin Oncol 2009;27:298-303. [Crossref] [PubMed]
- Cohn SL, Pearson AD, London WB, et al. The international neuroblastoma risk group (INRG) classification system: an INRG task force report. J Clin Oncol 2009;27:289-97. [Crossref] [PubMed]


