
Choosing high-risk screening vs. surgery and the effect of treatment modality on anxiety and breast-specific sensuality in BRCA mutation carriers
Introduction
Deleterious BRCA mutations are associated with 5–10% of all breast cancers (1,2). Also known as hereditary breast and ovarian cancer (HBOC), defects in homologous recombination associated with BRCA1 or 2 confer a 45–65% risk of developing breast cancer by age 70 (3,4). Hereditary breast cancers tend to develop earlier than non-hereditary breast cancers, with the average age of diagnosis in a BRCA1 mutation carrier being 42 years, compared to 62, the median age of diagnosis in the United States (5). High-risk screening (HRS) consisting of annual mammogram and MRI has been found to detect most breast cancers at a very early stage and is recommended to mBRCA who defer prophylactic mastectomy (6-8). However, up to a quarter of mBRCA report difficulty in deciding between prophylactic mastectomy and screening, and a third feel that prophylactic mastectomy is “too drastic” (9).
Choosing between HRS and prophylactic mastectomy has implications for psychosocial well-being, sexuality, and overall quality of life. Women with BRCA mutations who underwent therapeutic mastectomy reported poorer body image than non-carriers who underwent the same surgery (6). Furthermore, the effects of prophylactic mastectomy when compared to therapeutic mastectomy are mixed. In one prospective study of mBRCA undergoing prophylactic mastectomy with reconstruction, body image significantly worsened at 6 months postoperatively, and both body image and sexual satisfaction were lower at a median of 21 months postoperatively (7). In contrast, a long-term retrospective study of 609 women demonstrated favorable effects or no change in self-esteem, satisfaction with appearance, feelings of femininity, or sexual relationships at a mean follow-up of 14.5 years (8).
We have previously shown that patients undergoing surgery for breast cancer experience a postoperative decline in sexual function and breast-specific sensuality (10). It is less clear if mBRCA who undergo prophylactic mastectomy are equally as affected as those with a cancer diagnosis. If mBRCA who undergo mastectomy for breast cancer diagnosis are found to have significantly worse sexual function outcomes than those who undergo prophylactic mastectomy, then these outcomes should be shared with women faced with the decision to proceed with HRS or prophylactic mastectomy, as HRS does not decrease the risk of breast cancer, but only improves early diagnosis (11).
Sexual dysfunction has been correlated with higher levels of anxiety, and anxiety and depression often occur together (12,13). In patients who are diagnosed with a BRCA mutation, concerns about future cancer worry may play a role in choosing between HRS and prophylactic mastectomy. In the present study, we sought to compare sexual function, anxiety, and depression in mBRCA with and without breast cancer. We also sought to correlate sexual function outcomes and breast-specific sensuality to treatment decision-making in mBRCA. We hypothesized that mBRCA with breast cancer would have worse psychosocial and sexual function outcomes when compared to those without breast cancer, and this information could be used to encourage prophylactic surgery instead of HRS in mBRCA.
Methods
An anonymous, cross-sectional survey was approved for distribution by a single institution’s Institutional Review Board (IRBnet #819281). The researchers implemented all agreed procedures to maintain the confidentiality of the participant data as delineated in the IRB study protocol, and participation in the survey implied consent. BRCA1 and BRCA2 mutation carriers older than 18 years of age and fluent in the English language were included. All email addresses were collected from a single cancer center to patients who had undergone testing between October 1997 and February 2016. The survey was distributed electronically via REDCap electronic data capture tools from March 2016 until June 2016 (14). The survey link was emailed through a secure server using previously collected email addresses identified within the Women & Infants’ Hospital Cancer Genetics and Prevention Program’s database. Two additional reminder emails were sent regardless of completion status.
The survey tool had three parts: investigator-generated questions, the Female Sexual Function Index (FSFI), and the HADS survey. The 55 investigator-generated questions were designed to acquire patient demographic information such as age, education, marital status, household income, and sexual orientation. The investigators also collected data related to each patient’s current and prior cancer treatment, when applicable. See Supplement I for the complete survey. Without a validated method of measuring breast-specific sensuality, four questions previously developed and described were employed in the present study to capture patient’s assessments of the importance of the role of the chest in intimacy, as well as the perception of pleasurable caress before and after surgery (10).
The FSFI was also included. The FSFI is a 19-item tool measuring six domains of sexual function over the prior four weeks: desire, arousal, lubrication, orgasm, satisfaction, and pain. The survey has been validated for use in breast cancer patients (15). When comparing the FSFI with other commonly used instruments such as the Sexual Activity Questionnaire (SAQ), the FSFI includes self-stimulation and assesses activity during a clear timeframe and is therefore thought to be more sensitive to small changes in sexual activity. Sexual dysfunction has been identified as an FSFI score <26.55, with higher scores indicating higher levels of function (16). FSFI domain and total scores were not calculated for patients who did not report sexual activity over the past 4 weeks. Internal consistency for each domain in the study sample ranged from a Cronbach’s alpha of 0.91 for desire to 0.97 for lubrication.
The Hospital Anxiety and Depression Scale (HADS) is a 14-item questionnaire that assesses both anxiety and depression over the prior 7 days. The HADS has been validated in several languages as well as internationally to measure anxiety and depression in the outpatient setting. It has also been validated in patients with breast cancer. There are seven items that measure anxiety and seven measuring depression that are scored separately. Each domain has a maximum score of 21, with scores ≤7 being normal and higher scores being associated with more severe disease (17-20). Cronbach’s alpha was 0.89 for the anxiety scale and 0.80 for the depression scale.
Statistical analyses were performed using either Chi square or Fisher’s exact test for categorical variables and the Wilcoxon rank-sum or Kruskal-Wallis test for continuous variables. Ordinal categories were compared using the sign test for paired ordinal data. Two-tailed P values less than 0.05 were considered statistically significant. Respondents were analyzed in total and divided into two subgroups: those with and without a breast cancer diagnosis. Not all survey questions were answered by every respondent and this was reflected with a change in denominator noted in the results.
Results
Within our Breast Health Center, email addresses are collected during the registration process. Out of a database of 432 patients with validated BRCA mutations within our genetic counseling database, 170 valid email contacts were identified and received the electronic survey. After excluding one blank survey, one woman who did not report BRCA positivity, and two women who did not respond to the breast cancer question, surveys from sixty-three women were included (37% response rate). The median age was between 50 and 59 (range, 18–79). Approximately half had been diagnosed with breast cancer at the time of the survey. There was no significant difference in age distribution between those who did and did not have cancer (P=0.2), those who underwent HRS or bilateral mastectomy (P=0.9), and those who underwent lumpectomy or mastectomy for cancer (P=0.6). Most respondents (77.2%) were postmenopausal. Within this cohort, mBRCA with cancer were more likely to have undergone a prophylactic BSO (93.1% vs. 71.9%, P=0.046).
Sixty-seven percent of respondents were married and 80% had biological children. All participants were heterosexual. Only 15% reported that their BRCA mutation impacted their decisions regarding childbearing, and only 7% reported that they wanted more children but did not have any more because of their mutation. However, only 15 of the respondents were younger than the age of 40 at the time of their diagnosis. Among the 31 mBRCA with cancer, most (58.1%) were diagnosed with breast cancer between age 40–49. Thirteen of 31 (42%) discovered their breast cancer with self-exams, 1/31 (3%) were diagnosed by a doctor’s exam, and 12/31 (39%) were image-detected, whereas 5 (16%) had an occult breast cancer discovered at the time of prophylactic mastectomy.
Surgical decision-making in mBRCA without breast cancer
Only 20% of mBRCA without cancer had undergone prophylactic mastectomy in this population, and this was more common among BRCA1 mutation carriers when compared to BRCA2 mutation carriers (43% vs. 14%, P=0.035). Within this cohort, all prophylactic mastectomies were nipple-sparing mastectomies, and all underwent reconstruction. The mBRCA who had chosen prophylactic mastectomy tended to be younger than the HRS group. Table 1 outlines patient demographics divided by diagnosis and mutation management choice.
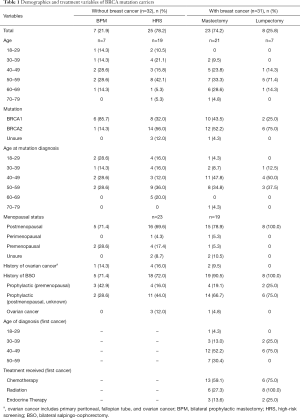
Full table
Eighty-six percent of respondents without breast cancer reported that they themselves played the biggest role in their decision regarding prophylactic mastectomy, which included all seven of those who eventually chose to undergo prophylactic mastectomy. Only one (3.5%) reported that her surgeon had the greatest influence on her decision.
Diagnosis and treatment of mBRCA with breast cancer
Among the mBRCA with breast cancer, more than half were diagnosed cancer between ages 40–49. Most breast cancers were diagnosed by breast exam (42%) or imaging (39%), while few (16%) were occult cancers discovered at the time of prophylactic mastectomy.
One quarter of the mBRCA with cancer chose to undergo lumpectomy and these patients were similar in age to those who chose mastectomy. Adjuvant treatment varied, with half receiving radiation (100% of lumpectomies and 27% of mastectomies), most (61%) receiving chemotherapy, and 5 of 30 (17%) eventually receiving endocrine therapy.
Anxiety and depression
Although most carriers reported that their mutation makes them anxious sometimes (63%) or often (19%), three-quarters of carriers were found to have normal HADS Anxiety and Depression Scores at the time of the survey. Among those without breast cancer, median HADS anxiety and depression scores were not different between those who chose prophylactic mastectomy or HRS (anxiety: 5 vs. 5, P=0.4; depression: 2 vs. 1, P=0.6), but all mBRCA who underwent prophylactic mastectomy reported they felt less anxious now that their breasts had been removed. In mBRCA with breast cancer, median HADS anxiety and depression scores were not different between those who underwent lumpectomy or mastectomy (anxiety: 5.5 vs. 6, P=0.9; depression: 3 vs. 2, P=0.6).
Breast specific sensuality and sexual function
More than half of all mBRCA (67% of those without cancer and 46% of those with cancer, P=0.019) reported that their chest played an important or somewhat important role in intimacy. Seventy-one percent of those without cancer and 100% of those with cancer reported a decline in pleasurable caress of the chest after mastectomy. Among those with cancer, there was a significant decline in the proportion of patients reporting pleasure with caress before (96%) compared to after (20%) surgery (P<0.001) (Table 2).
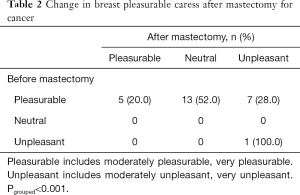
Full table
When comparing median total FSFI scores between study groups, they were not significantly different between patients with and without breast cancer (P=0.46), those who chose prophylactic mastectomy or HRS (P=0.25), and those who underwent mastectomy for cancer or prophylaxis (P=0.23). However, median total FSFI scores were below the cutoff for sexual dysfunction (<26.55) in the groups with breast cancer, those undergoing HRS, and those undergoing mastectomy for cancer (Table 3).
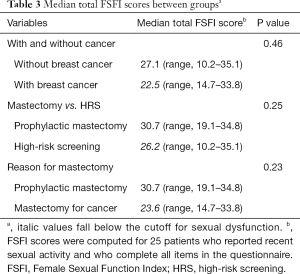
Full table
Among all carriers, FSFI satisfaction domain scores were strongly negatively correlated with both HADS anxiety and HADS depression scores (P=0.03, P=0.003). As shown in Table 4, FSFI and desire domain scores were also significantly negatively correlated with HADS depression score (P=0.01, P=0.02).
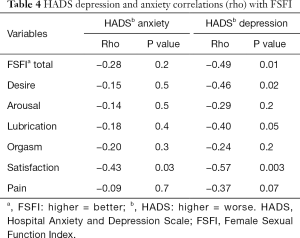
Full table
Discussion
Our study found that the role of the breast in intimacy is important to most patients with a BRCA mutation. Although most mBRCA reported a decline in breast-specific sensuality after mastectomy, those who underwent mastectomy for cancer reported a significant decline. Therefore, once a patient with a BRCA mutation is diagnosed with breast cancer, they may become more vulnerable to the derangements in breast-specific sensuality after mastectomy than their cancer-free counterparts. This finding has implications in the mBRCA decision making process: proceed with HRS, which does not decrease the risk of breast cancer, or prophylactic mastectomy, which does decrease the risk of breast cancer.
Interestingly, in mBRCA with breast cancer, median HADS anxiety and depression scores were not different between those who underwent lumpectomy or mastectomy. In a cohort study with a young population (median age 43, range, 27–82), those with BRCA-associated breast cancer who undergo lumpectomy have a risk of metachronous ipsilateral breast cancer that is similar to non-mBRCA counterparts of a similar age with a median follow-up of 76 months (21). In a more recent study of Dutch women, BRCA1 mutation carriers with breast cancer who received lumpectomy and radiation had a similar risk of local recurrence and overall survival compared to women who received mastectomy (22). Although long-term outcomes do not seem to be affected by surgical decision-making in this population, the majority of women who know their BRCA mutation status prior to surgery undergo unilateral or bilateral mastectomy (23). Women without a cancer diagnosis have been shown to have less cancer-related worry after risk-reducing mastectomy, but in the effect of breast conservation versus mastectomy in mBRCA with breast cancer is less clear and warrants further study (24-26).
Among those without cancer, anxiety scores were not different between those choosing prophylactic surgery or HRS. Lastly, FSFI scores, as expected, are negatively correlated with depression and anxiety in mBRCA. Although FSFI scores were not significantly different between those with and without cancer, this may have been a result of low statistical power. However, the median FSFI of mBRCA with cancer, those undergoing HRS, and those who underwent prophylactic mastectomy met criteria for sexual dysfunction.
Most carriers without cancer deferred prophylactic breast surgery, which was more pronounced in the BRCA2 population. The adoption of an HRS regimen incorporating annual mammogram and breast MRI in carriers may be driving these choices. Additionally, 44% of carriers were diagnosed with their mutation older than 50 years of age with age-adjusted risk estimates likely attenuating their cited breast cancer risk as it relates to preoperative counseling. Therefore, this population may be more likely to choose HRS instead of mastectomy than a younger population with a higher age-adjusted risk estimate. Anxiety scores were not different among women choosing to undergo HRS versus prophylactic mastectomy, which is likely due to self-selection biases. This result is in concordance with data suggesting no difference in cancer worry after risk-reducing surgery within one year of obtaining a BRCA positive result, when compared to preoperative cancer worry (27).
Almost three-quarters of mBRCA who underwent prophylactic mastectomy reported a decline in pleasurable caress of their reconstructed breasts, despite retaining their native nipple-areolar complex. This agrees with our previous work demonstrating that preservation of the native nipple-areolar complex may not translate to improved sexual function outcomes when compared to patients who do not retain their native nipple-areolar complex (28). The dramatic decline in pleasurable caress within this cohort further emphasizes the importance of preoperative counseling regarding the potential alterations in breast-specific sensuality after prophylactic surgery. Although breast cancer surgery, in general, has been associated with alterations in breast specific sensuality, patients who report improved appearance satisfaction postoperatively have been shown to have less sexual dysfunction as measured by the FSFI (10). The persistence of the importance of the role of the breast throughout prevention, diagnosis and treatment, as well as the post-treatment breast-specific changes in sexuality highlight the importance of the breast as a sensual organ.
Although more carriers with breast cancer met criteria for sexual dysfunction as measured by the FSFI when compared to those without a breast cancer diagnosis, we did not find that FSFI scores were significantly different between those without breast cancer who chose prophylactic mastectomy compared to those undergoing HRS. However, given the degree of difference, sample size may be limiting significance. We did find that sexual function as measured by the FSFI was negatively correlated with both depression and anxiety, highlighting that addressing depression and anxiety is a key component of managing sexual dysfunction.
Our findings were similar to Heiniger et al. who did not find a significant difference in anxiety or depression among high-risk women (as determined by the Tyrer-Cuzick algorithm) who underwent prophylactic mastectomy when compared to age and risk-matched controls, although their findings were likely also limited by self-selection bias. They did, however, report a reduction in cancer-related anxiety after surgery. Like our study, they reported no difference in sexual function between those who underwent prophylactic mastectomy when compared to those who did not, although their study employed the SAQ (29,30). Although a 2010 Swedish study found that prophylactic mastectomy can have a negative impact on body image and sexual enjoyment, a larger prospective study found no similar sexual function outcomes between high-risk women who underwent prophylactic mastectomy when compared to those who did not (24,31). More recently, our group looked at 453 women undergoing breast cancer surgery between 2000 and 2013 and found that surgical modality did not predict better or worse postoperative sexual function outcomes as measured by the FSFI. We did find that in lumpectomy patients, higher FSFI scores did result in improved appearance satisfaction and other parameters of breast-specific sensuality (10).
Our study population was slightly older (median age, 50–59) when compared to the median age of subjects in other studies examining the effect of genetic testing on surgical decision-making (32). Furthermore, among the study subjects who reported which mutation they carried, we had more BRCA2 mutation carriers compared to BRCA1.
This study was limited by its cross-sectional design. Preoperative psychological and sexual assessment was not available to the investigators. Therefore, the patient’s report of breast specific sensuality prior to surgery was subject to recall bias, which could have led to a more positive or negative assessment of their prior sexual function. Furthermore, the survey was completed once by each patient, instead of repeated over time, making it impossible to capture the changes in parameters with time. Although the large volume tertiary referral center where the study was executed facilitated the enrollment of subjects within a minority of the oncology patient population, our study was still limited by a 37% survey response rate, with even less patients completing the FSFI portion of the survey. This could be prevented in the future with a pre-study power analysis to set the targeted accrual, and also including multiple institutions in the eligible study cohort.
Our study did have several strengths. The survey was comprehensive and contained validated tools to measure sexual function, anxiety, and depression in cancer patients. It also included patients who had not undergone prophylactic surgery, which helped to characterize the differences between patients without cancer undergoing HRS versus prophylactic mastectomy.
With the integration of genetic testing into the diagnostic algorithm of many breast cancer patients, there are an increasing number of mBRCA identified. Women with BRCA mutations, sometimes diagnosed before menopause, are susceptible to derangements in sexual function during the course of both screening and treatment, and this appears to be correlated to both depression and anxiety. We found that mastectomy, prophylactic or therapeutic, negatively impacts breast specific sensuality and sexual function, although mBRCA with a breast cancer diagnosis may be more affected. Providers should be aware of these outcomes to offer early screening and intervention for these life-altering derangements that may be more pronounced in the treatment and not the prevention setting.
Supplementary
Supplement I
Hello,
You are receiving this anonymous survey because you have met with the genetic counsellors at the Women and Infants Breast Health Center in the past.
This is a study entitled “Sexual function, anxiety and surgical decision making in BRCA mutation carriers”. The main goal of this study is to look at how the surgical decisions made by BRCA mutation carriers, both for prevention and for treatment of breast cancer, affect anxiety levels and sexual function.
Using this survey, we are asking BRCA mutation carriers questions about their choices to undergo preventative surgery or continue heightened screening for breast cancer. For those women with a diagnosis of breast cancer, we would also like to know if they preferred a lumpectomy or mastectomy (removal of the breasts) with or without reconstruction. We will also ask questions relating to anxiety and recent sexual experience.
We hope the results of this study will aid physicians in guiding women who have tested positive for the BRCA gene mutation, in making surgical decisions for cancer treatment and prevention, as well as help prepare these women for the impact breast cancer treatment may have on their sexual experience.
This survey is
You do not have to take part in this study. You do not have to answer all the questions in the survey. If you feel uncomfortable at any time, you may skip the question or stop the survey all together. Should you need to talk to someone about your feelings we have a social worker, ******************** available to talk by phone at ******************** .
If you have any concerns or questions that come up while taking the survey, you may contact ********************************.
Thank you for your participation!
INSTRUCTIONS: Please answer the following questions as honestly and completely as possible. Your responses will be kept completely confidential and anonymous.
CHECK ONLY ONE BOX PER QUESTION:
- 1. Did you test positive for having either the BRCA1 or BRCA2 gene mutation?
- □ Yes
- □ No
- If you have answered “No” to question 1, end survey.
- 2. Which mutation did you test positive for?
- □ BRCA 1
- □ BRCA 2
- □ Uncertain
- 3. How many years ago did you discover you had a BRCA mutation?
- □ 0–1
- □ 1–3
- □ 3–5
- □ >5
- □ Uncertain
- 4. How old were you when they discovered your BRCA gene mutation?
- □ 18–29
- □ 30–39
- □ 40–49
- □ 50–59
- □ 60–69
- □ 70–79
- □ 80–89
- □ >90
- 5. Would you say that having the BRCA mutation makes you anxious?
- □ Often
- □ Sometimes
- □ Never
- 6. Have you ever been diagnosed with breast cancer?
- □ Yes
- □ No
- If you have answered “No” to question 6, move on to question 7.
- If you have answered, “Yes” to question 6, skip to question 23.
- 7. Have you had a double mastectomy (surgical removal of both breasts) for breast cancer prevention?
- □ Yes
- □ No
- 8. Who played the biggest role in your decision to have or not to have a double mastectomy?
- □ Self
- □ Partner/Spouse
- □ Surgeon
- □ Friend
- □ Family member
- □ Media
- □ Other
- If you have answered “No” to question 7, move on to question 9.
- If you have answered “Yes” to question 7, skip to question 12.
- 9. Do you want to or are you planning to have surgery to remove your breasts for breast cancer prevention?
- □ Yes
- □ No
- 10. Do you think that having surgery to remove your breasts will make you feel less anxious about someday getting breast cancer?
- □ Yes
- □ No
- 11. How pleasurable is it to have your breasts caressed or stimulated?
- □ Very pleasurable
- □ Moderately pleasurable
- □ Not pleasurable but not unpleasant
- □ Moderately unpleasant
- □ Very unpleasant
- 12. Do you undergo or have undergone in the past, regular screening with mammograms?
- □ Yes
- □ No
- 13. Do you undergo or have undergone in the past, regular screening with breast MRI?
- □ Yes
- □ No
- 14. Have you ever had a breast biopsy?
- □ Yes
- □ No
- If you have answered “Yes” to question 14, move on to question 15.
- If you have answered “No” to question 14, skip to question 16.
- 15. How many breast biopsies have you had?
- □ 1
- □ 1–2
- □ >2
- 16. Are you currently taking medication to prevent breast cancer like Tamoxifen or raloxifene (Evista) or anastrazole (Arimidex), aromasin (Exemestane), or letrozole (Femara)?
- □ Yes
- □ No
- If you have answered “Yes” to question 16, move on to question 17.
- If you have answered “No” to question 16, skip to question 18.
- 17. How long have you been taking this medication for?
- □ <5 years
- □ 5–10 years
- □ >10 years
- 18. How important of a role does your chest play in intimacy and sex for you?
- □ Very important
- □ Somewhat important
- □ Not very important
- □ No role at all
- If you have answered “Yes” to question 7, move on to question 19.
- If you have answered “No” to question 7, skip to question 41.
- 19. How pleasurable was it to have your breasts caressed or stimulated before you had surgery?
- □ Very pleasurable
- □ Moderately pleasurable
- □ Not pleasurable but not unpleasant
- □ Moderately unpleasant
- □ Very unpleasant
- 20. Do you feel less anxious about getting breast cancer now that your breasts have been removed?
- □ Yes
- □ No
- 21. Have you had breast reconstruction?
- □ Yes
- □ No
- If you have answered “Yes” to question 21, move on to question 22.
- If you answered “No” to question 21, skip to question 41.
- 22. How pleasurable is it to have your reconstructed breasts caressed or stimulated, now?
- □ Very pleasurable
- □ Moderately pleasurable
- □ Not pleasurable but not unpleasant
- □ Moderately unpleasant
- □ Very unpleasant
- 23. With breast reconstruction, did you keep your own nipples?
- □ Yes
- □ No
- The following questions are for women who have had breast cancer. If you have not had breast cancer, skip to question 41.
- 24. How was your breast cancer discovered?
- □ I felt it
- □ My doctor felt it
- □ Mammogram
- □ MRI
- □ Other
- 25. Did you have breast cancer once or more than once?
- □ Once
- □ More than once
- 26. How old were you when your first cancer was discovered?
- □ 18–29
- □ 30–39
- □ 40–49
- □ 50–59
- □ 60–69
- □ 70–79
- □ 80–89
- □ >90
- 27. What kind of surgery did you have for your first breast cancer?
- □ Lumpectomy (Removal of a lump)
- □ Mastectomy (Removal of one breast)
- □ Mastectomy with reconstruction (Removal of one breast with recreation of the breast)
- □ Double mastectomy without reconstruction
- □ Double mastectomy with reconstruction
- □ I did not have surgery
- For question 27, if you have answered “Mastectomy with reconstruction” or “Double mastectomy with reconstruction”, move on to question 28.
- For question 27, if you have answered “Lumpectomy”, “Mastectomy”, “Double mastectomy without reconstruction” or “I did not have surgery”, skip to question 29.
- 28. With breast reconstruction, did you keep your own nipples?
- □ Yes
- □ No
- 29. Did you have radiation for your first breast cancer?
- □ Yes
- □ No
- 30. Did you have chemotherapy for your first breast cancer?
- □ Yes
- □ No
- 31. Did you take endocrine therapy such as Tamoxifen, aromasin (Exemestane), letrozole (Femara) or anastrazole (Arimidex) for your first breast cancer?
- □ Yes
- □ No
- 32. How important of a role does your chest play in intimacy and sex for you?
- □ Very important
- □ Somewhat important
- □ Not very important
- □ No role at all
- 33. How pleasurable was it to have your breasts caressed or stimulated before you were diagnosed with breast cancer?
- □ Very pleasurable
- □ Moderately pleasurable
- □ Not pleasurable but not unpleasant
- □ Moderately unpleasant
- □ Very unpleasant
- 34. How pleasurable is it to have your treated/reconstructed breasts caressed or stimulated, now?
- □ Very pleasurable
- □ Moderately pleasurable
- □ Not pleasurable but not unpleasant
- □ Moderately unpleasant
- □ Very unpleasant
- If you have answered “Once” to question 25, move on to question 41.
- 35. How old were you when your second cancer was discovered?
- □ 18–29
- □ 30–39
- □ 40–49
- □ 50–59
- □ 60–69
- □ 70–79
- □ 80–89
- □ >90
- 36. What kind of surgery did you have for your second breast cancer?
- □ Lumpectomy (Removal of a lump)
- □ Mastectomy (Removal of one breast)
- □ Mastectomy with reconstruction (Removal of one breast with recreation of the breast)
- □ Double mastectomy without reconstruction
- □ Double mastectomy with reconstruction
- □ I did not have surgery
- For question 36, if you have answered “Mastectomy with reconstruction” or “Double mastectomy with reconstruction”, move on to question 37.
- For question 36, if you have answered “Lumpectomy”, “Mastectomy”, “Double mastectomy without reconstruction” or “I did not have surgery”, skip to question 38.
- 37. With breast reconstruction, did you keep your own nipples?
- □ Yes
- □ No
- 38. Did you have radiation for your second breast cancer?
- □ Yes
- □ No
- 39. Did you have chemotherapy for your second breast cancer?
- □ Yes
- □ No
- 40. Did you take endocrine therapy such as Tamoxifen, aromasin (Exemestane), letrozole (Femara) or anastrazole (Arimidex) for your second breast cancer?
- □ Yes
- □ No
- The following questions are for everyone taking this survey.
- 41. Have you ever had ovarian or fallopian tube cancer?
- □ Yes
- □ No
- 42. Have you had a salpingo-oophorectomy (fallopian tubes and ovaries surgically removed)?
- □ Yes
- □ No
- If you have answered “Yes” to question 42, move on to question 43.
- If you have answered “No” to question 42, move on to question 47.
- 43. Was your salpingo-oophorectomy done to prevent ovarian/fallopian tube cancer or to treat the cancer you already had?
- □ To prevent cancer
- □ To treat the cancer I already had
- If you have answered “To prevent cancer” for question 43, move on to question 44.
- If you have answered “To treat the cancer I already had” for question 43, skip to question 45.
- 44. Did you delay your preventative salpingo-oophorectomy so that you could have children?
- □ Yes
- □ No
- 45. What was your age when you had your salpingo-oophorectomy?
- □ 20–29
- □ 30–34
- □ 35–40
- □ 41–49
- □ 50–59
- □ >60
- 46. This question is about menopause. By menopause, we mean not having any periods for one year or more. What was your menopause status when your ovaries were removed?
- □ Post-menopausal
- □ Peri-menopausal (nearing menopause)
- □ Pre-menopausal
- □ Not sure
- 47. This question is about menopause. By menopause, we mean not having any periods for one year or more. What is your menopause status now?
- □ Post-menopausal
- □ Peri-menopausal (nearing menopause)
- □ Pre-menopausal
- □ Not sure
- If you have answered “Post-menopausal” or “Not sure” to question 47, move on to question 48.
- If you have answered “Peri-menopausal” or “Pre-menopausal” to question 47, skip to question 49.
- 48. Did you ever used hormone replacement therapy when you went through menopause after you had your fallopian tubes and ovaries removed preventatively?
- □ Yes
- □ No
- The next set of questions is about anxiety. Think about your BRCA mutation when answering these questions and how it has affected your life. These questions are for everyone taking this survey.
- 49. I feel tense or wound up….
- □ Most of the time
- □ A lot of the time
- □ From time to time, occasionally
- □ Not at all
- 50. I still enjoy the things I used to enjoy….
- □ Definitely as much
- □ Not quite so much
- □ Only a little
- □ Hardly at all
- 51. I get sort of frightened as if something awful is about to happen…
- □ Very definitely and quite badly
- □ Yes, but not too badly
- □ A little, but it doesn’t worry me
- □ Not at all
- 52. I can laugh and see the funny side of things….
- □ As much as I always could
- □ Not quite so much now
- □ Definitely not so much now
- □ Not at all
- 53. Worrying thoughts go through my mind…
- □ A great deal of the time
- □ A lot of the time
- □ From time to time but not too often
- □ Only occasionally
- 54. I feel cheerful…
- □ Not at all
- □ Not often
- □ Sometimes
- □ Most of the time
- 55. I can sit at ease and feel relaxed…
- □ Definitely
- □ Usually
- □ Not often
- □ Not at all
- 56. I feel as if I am slowed down…
- □ Nearly all the time
- □ Very often
- □ Sometimes
- □ Not at all
- 57. I get sort of frightened feeling like “butterflies” in the stomach…
- □ Not at all
- □ Occasionally
- □ Quite often
- □ Very often
- 58. I have lost interest in my appearance…
- □ Definitely
- □ I don’t take as much care as I should
- □ I may not take quite as much care
- □ I take just as much care as ever
- 59. I feel restless as I have to be on the move…
- □ Very much indeed
- □ Quite a lot
- □ Not very much
- □ Not at all
- 60. I look forward with enjoyment to things…
- □ As much as I ever did
- □ Rather less than I used to
- □ Definitely less than I used to
- □ Hardly at all
- 61. I get sudden feelings of panic…
- □ Very often indeed
- □ Quite often
- □ Not very often
- □ Not at all
- 62. I can enjoy a good book or radio or TV program…
- □ Often
- □ Sometimes
- □ Not often
- □ Very seldom
- The next 19 questions are from a tool that physicians use to evaluate a woman’s sexual function. These questions are for everyone taking this survey.
- Sexual desire or interest is a feeling that includes wanting to have a sexual experience, feeling receptive to a partner’s sexual initiation, and thinking or fantasizing about having sex.
- Sexual arousal is a feeling that includes both physical and mental aspects of sexual excitement. It may include feelings of warmth or tingling in the genitals, lubrication (wetness), or muscle contractions.
- Sexual activity can include caressing, foreplay, masturbation and vaginal intercourse.
- Sexual intercourse is defined as penile penetration (entry) of the vagina.
- Sexual stimulation includes situations like foreplay with a partner, self-stimulation (masturbation), or sexual fantasy
- 63. Over the past 4 weeks, how often did you feel sexual desire or interest?
- □ Almost always or always
- □ Most times (more than half the time)
- □ Sometimes (about half the time)
- □ A few times (less than half the time)
- □ Almost never or never
- 64. Over the past 4 weeks, how would you rate your level (degree) of sexual desire or interest?
- □ Very high
- □ High
- □ Moderate
- □ Low
- □ Very low or none at all
- 65. Over the past four weeks, have you engaged in sexual activity or intercourse?
- □ Yes
- □ No
- If you answered “Yes” to question 65, move on to question 66.
- If you answered “No” to question 65, skip to question 83.
- 66. Over the past 4 weeks, how often did you feel sexually aroused (“turned on”) during sexual activity or intercourse?
- □ Almost always or always
- □ Most times (more than half the time)
- □ Sometimes (about half the time)
- □ A few times (less than half the time)
- □ Almost never or never
- 67. Over the past 4 weeks, how would you rate your level of sexual arousal (“turn on”) during sexual activity or intercourse?
- □ Very high
- □ High
- □ Moderate
- □ Low
- □ Very low or none at all
- 68. Over the past 4 weeks, how confident were you about becoming sexually aroused during sexual activity or intercourse?
- □ Very high confidence
- □ High confidence
- □ Moderate confidence
- □ Low confidence
- □ Very low or no confidence
- 69. Over the past 4 weeks, how often have you been satisfied with your arousal (excitement) during sexual activity or intercourse?
- □ Almost always or always
- □ Most times (more than half the time)
- □ Sometimes (about half the time)
- □ A few times (less than half the time)
- □ Almost never or never
- 70. Over the past 4 weeks, how often did you become lubricated (“wet”) during sexual activity or intercourse?
- □ Almost always or always
- □ Most times (more than half the time)
- □ Sometimes (about half the time)
- □ A few times (less than half the time)
- □ Almost never or never
- 71. Over the past 4 weeks, how difficult was it to become lubricated (“wet”) during sexual activity or intercourse
- □ Extremely difficult or impossible
- □ Very difficult
- □ Difficult
- □ Slightly difficult
- □ Not difficult
- 72. Over the past 4 weeks, how often did you maintain your lubrication (“wetness”) until completion of sexual activity or intercourse?
- □ Almost always or always
- □ Most times (more than half the time)
- □ Sometimes (about half the time)
- □ A few times (less than half the time)
- □ Almost never or never
- 73. Over the past 4 weeks, how difficult was it to maintain your lubrication (“wetness”) until completion of sexual activity or intercourse?
- □ Extremely difficult or impossible
- □ Very difficult
- □ Difficult
- □ Slightly difficult
- □ Not difficult
- 74. Over the past 4 weeks, when you had sexual stimulation or intercourse, how often did you reach orgasm (climax)?
- □ Almost always or always
- □ Most times (more than half the time)
- □ Sometimes (about half the time)
- □ A few times (less than half the time)
- □ Almost never or never
- 75. Over the past 4 weeks, when you had sexual stimulation or intercourse, how difficult was it for you to reach orgasm (climax)?
- □ Extremely difficult or impossible
- □ Very difficult
- □ Difficult
- □ Slightly difficult
- □ Not difficult
- 76. Over the past 4 weeks, how satisfied were you with your ability to reach orgasm (climax) during sexual activity or intercourse?
- □ Very satisfied
- □ Moderately satisfied
- □ About equally satisfied and dissatisfied
- □ Moderately dissatisfied
- □ Very dissatisfied
- 77. Over the past 4 weeks, how satisfied have you been with the amount of emotional closeness during sexual activity between you and your partner?
- □ Very satisfied
- □ Moderately satisfied
- □ About equally satisfied and dissatisfied
- □ Moderately dissatisfied
- □ Very dissatisfied
- □ I do not have a partner
- 78. Over the past 4 weeks, how satisfied have you been with your sexual relationship with your partner?
- □ Very satisfied
- □ Moderately satisfied
- □ About equally satisfied and dissatisfied
- □ Moderately dissatisfied
- □ Very dissatisfied
- 79. Over the past 4 weeks, how often did you experience discomfort or pain during vaginal penetration?
- □ Almost always or always
- □ Most times (more than half the time)
- □ Sometimes (about half the time)
- □ A few times (less than half the time)
- □ Almost never or never
- 80. Over the past 4 weeks, how often did you experience discomfort or pain following vaginal penetration?
- □ Almost always or always
- □ Most times (more than half the time)
- □ Sometimes (about half the time)
- □ A few times (less than half the time)
- □ Almost never or never
- 81. Over the past 4 weeks, how would you rate your level (degree) of discomfort or pain following vaginal penetration?
- □ Very high
- □ High
- □ Moderate
- □ Low
- □ Very low or none at all
- 82. Over the past 4 weeks, how satisfied have you been with your overall sexual life?
- □ Very satisfied
- □ Moderately satisfied
- □ About equally satisfied and dissatisfied
- □ Moderately dissatisfied
- □ Very dissatisfied
- The following questions involve demographic information and are for everyone taking this survey.
- 83. How would you choose to label your ethnicity? (Choose all that apply)
- □ American Indian or Alaska Native
- □ Asian
- □ Black or African American
- □ Hispanic
- □ Native Hawaiian or Other Pacific Islander
- □ White
- □ Other
- 84. What is your marital status?
- □ Single
- □ Married
- □ Divorced
- □ Widowed
- □ Separated
- 85. Do you have sexual relations with men, women or both?
- □ Only men
- □ Only women
- □ Both men and women
- 86. Do you have children?
- □ Yes
- □ No
- If you answered “Yes” to question 86, move onto question 87.
- If you answered “No” to question 86, move on to question 92.
- 87. How many children do you currently have?
- □ 1
- □ 2
- □ 3
- □ 4
- □ >4
- 88. Did you/do you want more children but have not had any more because of your BRCA mutation?
- □ Yes
- □ No
- 89. When you were diagnosed with a BRCA mutation, had you already had children?
- □ Yes
- □ No
- 90. Did you breastfeed?
- □ Yes
- □ No
- 91. Did breastfeeding impact your decision regarding timing of prophylactic (preventative) mastectomy?
- □ Yes
- □ No
- □ I did not have a prophylactic (preventative) mastectomy
- 92. Has/was the option of genetic testing of an embryo for the BRCA mutation, with in-vitro fertilization (IVF) to prevent your unborn child from getting the BRCA mutation, ever discussed with you?
- □ Yes
- □ No
- 93. Did you/do you have anxiety regarding the timing of your prophylactic breast or ovarian surgery with respect to childbearing?
- □ Yes
- □ No
- 94. Did/does your BRCA mutation impact your decisions regarding childbearing?
- □ Yes
- □ No
- 95. What is your age?
- □ 18–29
- □ 30–39
- □ 40–49
- □ 50–59
- □ 60–69
- □ 70–79
- □ 80–89
- □ >90
- 96. What is the highest degree or level of school you have completed? (If currently enrolled, highest degree received)?
- □ Some high school
- □ High school graduate
- □ Trade/technical/vocational training
- □ Some college, no diploma
- □ Bachelor’s degree
- □ Master’s degree
- □ Doctoral degree
- 97. What is your average household income?
- □ <$20,000/year
- □ $20,000 to $39,999/year
- □ $40,000 to $59,999/year
- □ $60,000 to $79,999/year
- □ $80,000 to $99,999/year
- □ $100,000 to $199,999/year
- □ $>200,000/year
- □ Rather not answer
Thank you for participating in our survey!
We understand that many of the questions were very personal. You can be assured that your answers remain completely anonymous.
At this time, if you feel the need to speak with a social worker, please do not hesitate to contact ********************.
If you have any other concerns or questions that came up while taking the survey, you may also contact ********************.
Thank you again and have a wonderful day!
Acknowledgments
We would like to thank the staff of the Women & Infants’ Breast Health Center that helped execute this study. We would also like to thank our patients, without whom this study would not be possible. There was no financial support for this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: An anonymous, cross-sectional survey was approved for distribution by a single institution’s Institutional Review Board (IRBnet #819281). The researchers implemented all agreed procedures to maintain the confidentiality of the participant data as delineated in the IRB study protocol, and participation in the survey implied consent.
References
- Campeau PM, Foulkes WD, Tischkowitz MD. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Hum Genet 2008;124:31-42. [Crossref] [PubMed]
- Easton DF. How many more breast cancer predisposition genes are there? Breast Cancer Res 1999;1:14-17. [Crossref] [PubMed]
- Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003;72:1117-30. [Crossref] [PubMed]
- Rojas K, Stuckey A. Breast cancer epidemiology and risk factors. Clin Obstet Gynecol 2016;59:651-72. [Crossref] [PubMed]
- Brose MS. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst 2002;94:1365. [Crossref] [PubMed]
- van Oostrom I, Meijers-Heijboer H, Lodder L, et al. Long-term psychological impact of carrying a BRCA 1/2 mutation and prophylactic surgery: a 5-year follow-up study. J Clin Oncol 2003;21:3867-74. [Crossref] [PubMed]
- Gopie JP, Mureau MA, Seynaeve C, et al. Body image issues after bilateral prophylactic mastectomy with breast reconstruction in health women at risk for hereditary breast cancer. Fam Cancer 2013;12:479-87. [Crossref] [PubMed]
- Frost MH, Schaid DJ, Sellers TA, et al. Long-term satisfaction and psychological social function following bilateral prophylactic mastectomy. JAMA 2000;284:319-24. [Crossref] [PubMed]
- Litton JK, Westin SN, Ready K, et al. Perception of screening and risk reduction surgeries in patients tested for a BRCA deleterious mutation. Cancer 2009;115:1598-604. [Crossref] [PubMed]
- Gass JS, Onstad M, Pesek S, et al. Breast-Specific Sensuality and Sexual Function in Cancer Survivorship: Does Surgical Modality Matter? Ann Surg Oncol 2017;24:3133-40. [Crossref] [PubMed]
- Passaperuma K, Warner E, Causer PA, et al. Long-term results of screening with magnetic resonance imaging in women with BRCA mutations. Br J Cancer 2012;107:24-30. [Crossref] [PubMed]
- Kowalczyk R, Cedrych I, Lew-Starowicz Z, et al. Predictors of sexual function in women after treatment for breast cancer. Journal Clin Oncol 2016;34:10071. [Crossref]
- Listøl W, Høberg-Vetti H, Eide GE, et al. Anxiety and depression symptoms among women attending group-based education courses for hereditary breast and ovarian cancer. Hereditary Cancer in Clinical Practice 2017;15:2. [Crossref] [PubMed]
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. [Crossref] [PubMed]
- Bartula I, Sherman K. The female sexual functioning index (FSFI): evaluation of acceptability, reliability, and validity in women with breast cancer. Support Care Cancer 2015;23:2633-41. [Crossref] [PubMed]
- Baser RE, Li Y, Carter J. Psychometric validation of the female sexual function index (FSFI) in cancer survivors. Cancer 2012;118:4606-18. [Crossref] [PubMed]
- Stern AF. The Hospital Anxiety and Depression Scale. Occup Med (Lond) 2014;64:393-4. [Crossref] [PubMed]
- Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77. [Crossref] [PubMed]
- Herrmann C. International experiences with the Hospital Anxiety and Depression Scale--a review of validation data and clinical results. J Psychosom Res 1997;42:17-41. [Crossref] [PubMed]
- Osborne RH, Elsworth GR, Sprangers MA, et al. The value of the Hospital Anxiety and Depression Scale (HADS) for comparing women with early onset breast cancer with population-based reference women. Qual Life Res 2004;13:191-206. [Crossref] [PubMed]
- Robson M, Svahn T, McCormick B, et al. Appropriateness of breast-conserving treatment of breast carcinoma in women with germline mutations in BRCA1 or BRCA2. Cancer 2005;103:44-51. [Crossref] [PubMed]
- van den Broek AJ, Schmidt MK, van 't Veer LJ, et al. Prognostic impact of breast-conserving therapy versus mastectomy of BRCA1/2 mutation carriers compared with noncarriers in a consecutive series of young breast cancer patients. Ann Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Park S, Lee J, Ryu J, et al. Genetic diagnosis before surgery has an impact on surgical decision in BRCA mutation carriers with breast cancer. World J Surg 2018;42:1384-90. [Crossref] [PubMed]
- Hatcher MB, Fallowfied L, A’Hern R. The psychosocial impact of bilateral prophylactic mastectomy: prospective study using questionnaires and semistructured interviews. BMJ (Clinical Research Ed) 2001;322:76. [Crossref] [PubMed]
- Haroun I, Graham T, Poll A, et al. Reasons for risk-reducing mastectomy versus MRI-screening in a cohort of women at high hereditary risk of breast cancer. Breast 2011;20:254-8. [Crossref] [PubMed]
- Kwong A, Chu A. What made her give up her breasts: a qualitative study on decisional considerations for contralateral prophylactic mastectomy among breast cancer survivors undergoing BRCA1/2 genetic testing. Asian Pac J Cancer Prev 2012;13:2241-7. [Crossref] [PubMed]
- Watson M, Foster C, Eeles R, et al. Psychosocial impact of breast/ovarian (BRCA1/2) cancer-predictive genetic testing in a UK multi-centre clinical cohort. Brit J Cancer 2004;91:1787-94. [Crossref] [PubMed]
- Rojas K, Onstad M, Raker C, et al. The impact of mastectomy type on the Female Sexual Function Index (FSFI), satisfaction with appearance, and the reconstructed breast's role in intimacy. Breast Cancer Res Treat 2017;163:273-9. [Crossref] [PubMed]
- Heiniger L, Butow P, Coll J, et al. Long-term outcomes of risk-reducing surgery in unaffected women at increased familial risk of breast and/or ovarian cancer. Fam Cancer 2015;14:105-15. [Crossref] [PubMed]
- Tyrer J, Duffy S. Cuzick. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004;23:1111-30. [Crossref] [PubMed]
- Gahm J, Wickman M, Brandber Y. Bilateral prophylactic mastectomy in women with inherited risk of breast cancer- prevalence of pain and discomfort, impact on sexuality, quality of life and feelings of regret two years after surgery. Breast 2010;19:462-9. [Crossref] [PubMed]
- Yadav S, Reeves S, Campian S, et al. preoperative genetic testing impacts surgical decision making in BRCA mutation carriers with breast cancer: a retrospective cohort. Hered Cancer Clin Pract 2017;15:11. [Crossref] [PubMed]


