
Diagnostic value of major salivary gland ultrasonography in primary Sjögren’s syndrome: the role of grey-scale and colour/power Doppler sonography
Introduction
Sjogren’s syndrome (SS) is a systemic autoimmune disease whose main target is the exocrine glands, mainly the salivary and tear glands (1). The term primary SS (pSS) refers to those conditions that are not associated with other connective tissue diseases, particularly rheumatoid arthritis. It mainly affects middle-aged women, with an estimated prevalence between 0.02% and 0.1% (2). More rarely it concerns men, the elderly or children.
The clinical presentation of pSS can be heterogeneous, ranging from symptoms of oral and ocular dryness, to systemic and multi-organ disease, to a condition that predisposes to the onset of lymphoproliferative disorders (1,3). The pathophysiological mechanism underlying pSS is the immune-mediated destruction of the epithelium of the exocrine glands, involving B and T cell responses. Classically, autoantibody biomarkers are directed against the antigens Ro/SSA and La/SSB. The diagnostic criteria for pSS include the presence of autoantibodies in the serum, but the diagnostic cornerstone remains histological analysis of the tissue of the minor salivary glands (4). Salivary gland ultrasonography (SGUS) is a recently introduced imaging technique to evaluate the involvement of the major salivary glands in pSS. Over the past few years, SGUS has received some interest, as it does not use ionizing radiation, is non-invasive, and is easily performed in daily clinical practice (5). According to the criteria of the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) (criteria ACR/EULAR), some patients without sicca symptoms may be classified as suffering from pSS (4). The inclusion of SGUS in the ACR/EULAR criteria would seem to improve its sensitivity from 64.4% to 84.4%, without changing its specificity (89.3% vs. 91.0%) (6). According to some researchers, SGUS may soon lead to the replacement of more complex techniques such as sialography and salivary scintigraphy (7,8).
SGUS as a diagnostic tool for pSS
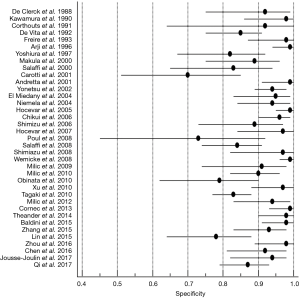
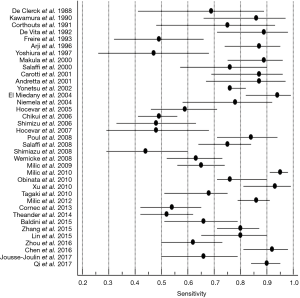
SGUS allows to identify ecostructural anomalies characteristic of the disease (7,9-11) and its high sensitivity compared to other methods has already been demonstrated (7,11,12). The diagnostic accuracy of SGUS is also high in the early stages of pSS (13-18). In this review we identified 37 studies that examined the properties of SGUS for the diagnosis of pSS. Most of these contributions used a case-control design. A meta-analysis revealed that the common denominator of the studies is high specificity (pooled specificity 0.91%; 95% CI: 0.88–0.93%) (Figure 1), demonstrating how SGUS successfully reveals unaffected subjects. On the other hand, sensitivity was also considerably high (pooled sensitivity 0.83%; 95% CI: 0.78–0.87%) (Figure 2) (18). The main limitation in the interpretation of the pooled data is however represented by the low quality of the studies included and their clinical and methodological heterogeneity.
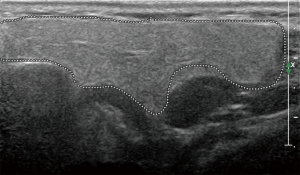
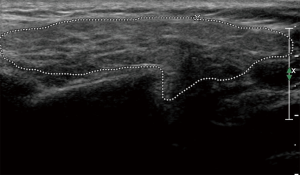
Echostructural abnormalities detectable on SGUS
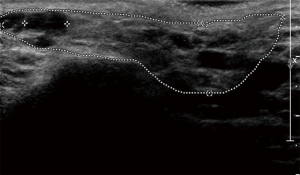
The principal ecostructural abnormalities detectable by SGUS are the following: parenchymal non-homogeneity, hypo-anechoic or hyperchoic areas (due respectively to cysts or calcifications), size variations, irregularities in glandular profiles and the presence of intra- or periglandular lymph nodes (19) (Figures 3-5). As highlighted by several authors (9,20), the most relevant SGUS alteration of pSS is the parenchymal inhomogeneity detectable bilaterally. This ultrasound finding, is the one that has shown the greatest agreement with the alterations documented with the scintigraphy of the salivary glands, sialography, and minor labial salivary gland biopsy (LSGB) (21,22). At the level of the parotid and submandibular glands, SGUS shows the best relationship of sensitivity to specificity, with a positive predictive value of 72.0% and a negative predictive value of 96.0% (23). Compared to contrast sialography and to salivary gland scintigraphy, SGUS showed higher sensitivity (75.3%, 72.7% and 70.1%, respectively), with similar specificity (83.5%, 84.9% and 82.3%, respectively) (7). Ultrasound findings of hypoechoic, multiple, circumscribed or confluent areas and/or multiple cysts correspond to a histological pattern of ductal ectasia surrounded by lymphocyte infiltrate or dilated glandular lobes surrounded by lymphocyte aggregates. In particular, Kawamura et al. (24) and Salaffi et al. (7,12) demonstrated that the anomalies documented at SGUS are strongly related to histological changes (12), and that the SGUS score proposed for classification is well correlated with sialographic classifications (12). Cornec et al. (15) have verified that morphological abnormalities of the salivary glands can be detected early in the course of pSS. The diagnostic characteristics of the SGUS also seem not to vary during the disease. Applying an ultrasound cut-off of 5, the proposed SGUS scoring system was slightly less specific (85.7% vs. 77.9%) but more sensitive (94.9% vs. 98.7%) compared to the AECG criteria (4,13).
Comparison of SGUS semiquantitative scoring systems
To date, several scoring systems are available in the literature for assessing the severity pSS on the basis of SGUS. In a meta-analysis, Delli et al. have identified 33 scoring systems used to assess the involvement of the major salivary glands in the course of pSS (18). Among them, the scoring systems are rather heterogeneous, and this heterogeneity is related to several factors: type of salivary glands examined, ultrasound features evaluated, and cut-off applied. Hocevar et al. have defined a methodology widely used in several contributions (9,10,25-27). This method dates back to 2005, and is based on five components (erogeneity scored from 0 to 1, homogeneity, presence of hypoechoic areas, presence of hyperechoic reflexes, and clarity of the edges of the glands scored from 0 to 3) with a sensitivity of 58.8%, and a specificity of 98.7%. However, this scoring system is time-consuming and difficult to apply in daily clinical practice. Consequently, over the years the literature is proposing simpler scoring systems (15,20,28,29).
Alongside the Hocevar scoring system (9), the most widely used systems have been developed by De Vita et al. (30), whose system is the oldest available in literature, by Salaffi et al. (7), and by Milic et al. (10). The scoring system of De Vita et al. (30) dates back to 1992, and has been developed to define in a simplified way the parenchymal structural anomalies on the basis of a semi-quantitative score from 0 to 3: from normal to marked parenchymal inhomogeneity.
In 2008, Salaffi et al. (7) modified the De Vita scoring system (30), evaluating different hypo- or anechoic areas in different glands. This scoring system summarizes the changes of SGUS in each of the four main salivary glands, recording the alterations of these ultrasound findings: parenchymal homogeneity, echogenicity, gland size, posterior glandular boundary. Each of these parameters was evaluated according to the scoring system described in Table 1. The final score ranges from 0 to 16, obtained by adding the scores [0–4] for each parotid and submandibular gland. A pathological pattern is defined by a score of at least 1 in both parotids or in both submandibulars (grade 1). Grade 2 is a clear parenchymal inhomogeneity, characterized by multiple hypoechogenic areas of varying sizes (<2 mm), distributed unevenly. Using a cut-off of 6, the best ratio between sensitivity (75.3%) and specificity (83.5%) was obtained, with a positive likelihood ratio of 4.58. Applying a cut-off of 8 gains in specificity, but pays for a clear loss of sensitivity (sensitivity 54.5%, specificity 97.5%, positive likelihood ratio 21.5) (7). Milic et al. (10) proposed in 2010 a total ultrasound scoring system between 0 and 48 (0–12 for each gland), revealing a sensitivity of 95.1% and a specificity of 98.1%.

Full table
The wide variability in the proposed scores and cut-offs led to a great diversity of sensitivity and specificity in the available scoring systems (18). Table 1 summarises the main characteristics of the four scoring systems mentioned above.
Reproducibility of SGUS in pSS
Few researches have evaluated the reproducibility of SGUS in pSS. In the study by Salaffi et al. (7) the agreement measured on blind evaluations performed by two radiologists was high, with a kappa of 0.88 for the submandibular glands and 0.93 for the parotid glands respectively. Hocevar et al. (9) also documented an excellent interobserver agreement (0.90 kappa) with two blind operators. Both in the evaluation of echogenicity, inhomogeneity and presence of hypoechoic areas it is possible to document an excellent interobserver agreement (k =0.88, 0.90 and 0.88, respectively). El Miedany et al. (31) confirmed an excellent agreement (kappa 0.8 for the parotid gland) in assessing intraobserver reproducibility. In order to evaluate interobserver reproducibility, Salaffi et al. (7) studied patients with sicca symptoms without pSS or with pSS, obtaining a kappa of 0.85 for parenchymal homogeneity, 0.82 for echogenicity, 0.80 for gland size and posterior glandular boundary clarity. Interobserver reproducibility was lower than the overall score, suggesting the importance of establishing a common method for measuring scores. The reliability of interobserver readings of the submandibular glands was lower: 0.46 for homogeneity and 0.38 for echogenicity. Some authors (27), in order to increase the feasibility of ultrasound examination in daily clinical practice, proposed to evaluate the hypoechoic areas in a parotid and submandibular gland. However, in our opinion, it is of fundamental importance to carry out an ultrasound examination of all four major salivary glands in order to document the presence of neoformations or lithiasis.
Probably the scoring defined by Jousse-Joulin et al. (17) seems to have the best balance between simplicity and diagnostic accuracy. This scoring system also has its limits, in particular cysts are considered the only criterion of heterogeneity of the glandular parenchyma with respect to fat involution, a rather frequent condition in the submandibular glands. Fat involution can compromise the ability to visualize the salivary gland correctly. With fat involution, the edges of the glands become poorly defined and numerous thick hyperechoic bands can be seen.
SGUS as a prognostic tool
The possibility of performing outcome measurements and prognostic predictions in patients with pSS is very important for patients with pSS in view of the fact that the disease may evolve into severe extraglandular manifestations such as lymphoma.
Repeated SGUS may be useful for the early diagnosis of this serious complication, but also for highlighting possible therapeutic outcomes (32,33).
Theander et al. (28) investigated the prognostic value of SGUS during pSS. These authors observed hypoechoic lesions in 52% of pSS patients versus 1.8% of controls (P<0.001), with a specificity and a positive predictive value both of 98.0%, while sensitivity and negative predictive values were 52.0% and 53.0% respectively. Subjects with a pathological SGUS had more frequently significant systemic complications, increased disease activity and markers of lymphoma development, such as skin vasculitis, salivary gland swelling, detection of central germ structures in LSGB, and CD4+ T cell lymphopenia. Therefore, ultrasound parenchymal inhomogeneity may add real-time information useful in characterizing patients at high and low risk of complications. Lee et al. (34) confirmed the high sensitivity and specificity of SGUS in distinguishing patients with pSS from those with idiopathic sicca syndrome, also revealing in a linear regression model that the combination of SGUS positive and the presence of anti-SSA/Ro antibodies optimally predicts the classification of pSS according to the AECG, ACR and ACR-EULAR criteria (27). Astorri et al. (35) have also demonstrated that SGUS can stratify patients who are extractable nuclear antibodies (ENA)-negative: in ENA-negative patients without sonographic signs of pSS, LSGB should not be performed, as it is unlikely that it will add further information.
Conversely, some authors argue that in ENA-positive patients, LSGB should be performed independently of the results obtained by SGUS to study the presence of ectopic structures similar to germination centers and to diagnose the most severe phenotype of pSS (36). In the comparison between SGUS and histopathology, a clear role is proposed for SGUS in the stratification of ENA-negative patients by reducing the number of useless LSGBs. El Miedany et al. (31) evaluated the use of SGUS as a predictor of LSGB histopathological score: the ultrasound score was significantly correlated with the histopathological score (r=0.82). There was a high correlation between SGUS and LSGB grading (r=0.84) (12). The receiver operating characteristic curves (ROC) showed the good diagnostic properties of SGUS, followed by the semi-quantitative score of focus on LSGB. Attempts to evaluate the independent contribution of fractional LSGB composition since the predictor of the SGUS score showed a significant contribution of both fractional composition of inflammatory infiltrates and intralobular ducts. These results suggest that the ultrasonographic findings are strongly related to pSS (12).
Colour/power Doppler sonography (C/PDUS) of the salivary glands
Research has also focused heavily on the field of color/power Doppler US (C/PDUS) despite the fact that the salivary glands have a complex vascularization. With the C/PDUS technique, a hemodynamic study of the glands can be carried out and the vascularization characteristics of a possible lesion evaluated. The C/PDUS is a complementary technique to the ultrasound B-mode study, and with it we can analyze both the physiological changes that occur during saliva stimulation in normal subjects (37) and the alteration of blood flow in the pathological glands (19,38,39). In patients with pSS a glandular hypervascularisation has been described. Hypervascularization is generally a diffused pattern, deriving from the presence of small vessels, both peripheral and randomly distributed inside the glands, visible as punctiform signals. The hypervascular pattern seemed to be directly related to the extent of parenchymal changes, being greater in the glands with higher parenchymal heterogeneity and higher number of cystiform structures. It may also be interesting to evaluate the resistivity and pulsatility indices of the facial artery which are decreased in patients with pSS, with a less intense response to lemon juice stimulation in patients than in controls (37,39). Hypervascularisation may result in a reduction in the resistive index of the facial artery. Martinoli et al. (37) used the Doppler technique to evaluate the vascular anatomy of the salivary glands and the changes induced by lemon juice stimulation. Stimulation leads to a marked increase in colour signals within the glandular parenchyma and to the development of an aliasing artifact due to an increased flow rate; conversely, arterial impedance is decreased resulting in a decrease in the resistive index (37). The values of the resistive index seem to return to normal within 20 seconds of stimulation with lemon juice (37). The hypervascular pattern had already been described in the course of autoimmune diseases with exocrine glandular involvement (40). It probably represents a common and unspecific finding: hyperemia is associated with inflammation in this class of diseases. Lee and co-workers (34) documented reduced volumes in the parotid and submandibular glands, and the presence of a reduced C/PDUS signal in patients with advanced pSS. Hypervascularisation and increased glandular volume would be characteristic of the inflammatory and early phase of pSS, while a reduction in glandular volume associated with hypo-vascularisation are characteristic glandular late stages. Consequently, increasing the C/PDUS signal without definite SGUS structural changes might be characteristic of the early stages of pSS. Chikui et al. (39), dealing with vascularization indices, revealed that the waveform of patients with pSS was more uniform than in healthy subjects, corroborated by a reduction in resistivity and pulsatility indices, suggesting the presence of downstream hypervascularisation. After stimulation of salivary secretion, the facial artery of healthy subjects changed decreasing the resistive and pulsatility indices, waveform changes indicative of increased blood flow to the submandibular gland. In contrast, the facial artery of patients with pSS did not respond sufficiently to stimulation, showing changes in resistivity and pulsatility indices significantly lower than those of controls. Doppler waveform abnormalities are related to the severity of glandular damage, indicating a close connection between altered blood flow of the salivary gland and altered secretory function in pSS. Carotti et al. (41) documented that peak systolic velocity was more sensitive than the resistive index. Resistivity values of the parotids and submandibular glands did not show significant changes after lemon stimulation in patients with pSS or in controls.
Conclusions
In conclusion, ultrasound examination of the salivary glands contributes significantly to the diagnosis of pSS. The main advantages are the prompt availability, repeatability, and low cost of the method. In addition, all major salivary glands can be easily studied in a single examination, and it is also possible to identify a proportion of subjects at greater risk of extra-glandular complications. However, in the context of early detection of pSS, and probably for follow-up monitoring of the disease in the context of clinical trials and in the context of lymphoproliferative disease, more sophisticated scoring systems, including vascularization characteristics, will be needed. Within the imaging methods available for pSS, SGUS integrated with the C/PDUS technique is considered to be the first-line examination for both diagnosis and follow-up.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brito-Zerón P, Baldini C, Bootsma H, et al. Sjögren syndrome. Nat Rev Dis Primers 2016;2:16047. [Crossref] [PubMed]
- Maciel G, Crowson CS, Matteson EL, et al. Prevalence of primary Sjögren’s syndrome in a population-based cohort in the United States. Arthritis Care Res (Hoboken) 2017;69:1612-6. [Crossref] [PubMed]
- Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med 2004;164:1275-84. [Crossref] [PubMed]
- Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 2017;69:35-45. [Crossref] [PubMed]
- Takagi Y, Sumi M, Nakamura H, et al. Ultrasonography as an additional item in the American College of Rheumatology classification of Sjögren's syndrome. Rheumatology (Oxford) 2014;53:1977-83. [Crossref] [PubMed]
- Cornec D, Jousse-Joulin S, Marhadour T, et al. Salivary gland ultrasonography improves the diagnostic performance of the 2012 American College of Rheumatology classification criteria for Sjögren’s syndrome. Rheumatology (Oxford) 2014;53:1604-7. [Crossref] [PubMed]
- Salaffi F, Carotti M, Iagnocco A, et al. Ultrasonography of salivary glands in primary Sjogren’s syndrome: a comparison with contrast sialography and scintigraphy. Rheumatology (Oxford) 2008;47:1244-9. [Crossref] [PubMed]
- Vitali C, Carotti M, Salaffi F. Is it the time to adopt salivary gland ultrasonography as an alternative diagnostic tool for the classification of patients with Sjogren’s syndrome? Arthritis Rheum 2013;65:1950. [Crossref] [PubMed]
- Hocevar A, Ambrozic A, Rozman B, et al. Ultrasonographic changes of major salivary glands in primary Sjogren’s syndrome. Diagnostic value of a novel scoring system. Rheumatology (Oxford) 2005;44:768-72. [Crossref] [PubMed]
- Milic VD, Petrovic RR, Boricic IV, et al. Diagnostic value of salivary gland ultrasonographic scoring system in primary Sjogren’s syndrome: a comparison with scintigraphy and biopsy. J Rheumatol 2009;36:1495-500. [Crossref] [PubMed]
- Makula E, Pokorny G, Rajtar M, et al. Parotid gland ultrasonography as a diagnostic tool in primary Sjogren’s syndrome. Br J Rheumatol 1996;35:972-7. [Crossref] [PubMed]
- Salaffi F, Argalia G, Carotti M, et al. Salivary gland ultrasonography in the evaluation of primary Sjogren’s syndrome. Comparison with minor salivary gland biopsy. J Rheumatol 2000;27:1229-36. [PubMed]
- Billings M, Amin Hadavand M, Alevizos I. Comparative analysis of the 2016 ACR-EULAR and the 2002 AECG classification criteria for Sjögren's syndrome: Findings from the NIH cohort. Oral Dis 2018;24:184-90. [Crossref] [PubMed]
- Baldini C, Luciano N, Tarantini G, et al. Salivary gland ultrasonography: a highly specific tool for the early diagnosis of primary Sjögren's syndrome. Arthritis Res Ther 2015;17:146. [Crossref] [PubMed]
- Cornec D, Jousse-Joulin S, Pers JO, et al. Contribution of salivary gland ultrasonography to the diagnosis of Sjögren’s syndrome: toward new diagnostic criteria? Arthritis Rheum 2013;65:216-25. [Crossref] [PubMed]
- Hammenfors DS, Brun JG, Jonsson R, et al. Diagnostic utility of major salivary gland ultrasonography in primary Sjögren’s syndrome. Clin Exp Rheumatol 2015;33:56-62. [PubMed]
- Jousse-Joulin S, Milic V, Jonsson MV, et al. Is salivary gland ultrasonography a useful tool in Sjögren’s syndrome? A systematic review. Rheumatology (Oxford) 2016;55:789-800. [Crossref] [PubMed]
- Delli K, Dijkstra PU, Stel AJ, et al. Diagnostic properties of ultrasound of major salivary glands in Sjögren's syndrome: a meta-analysis. Oral Dis 2015;21:792-800. [Crossref] [PubMed]
- Carotti M, Ciapetti A, Jousse-Joulin S, et al. Ultrasonography of the salivary glands: the role of grey-scale and colour/power Doppler. Clin Exp Rheumatol 2014;32:S61-70. [PubMed]
- Wernicke D, Hess H, Gromnicaihle E, et al. Ultrasonography of salivary glands – A highly specific imaging procedure for diagnosis of Sjögren’s syndrome. J Rheumatol 2008;35:285-93. [PubMed]
- Niemelä RK, Takalo R, Pääkkö E, et al. Ultrasonography of salivary glands in primary Sjögren's syndrome. A comparison with magnetic resonance imaging and magnetic resonance sialography of parotid glands. Rheumatology (Oxford) 2004;43:875-9. [Crossref] [PubMed]
- Niemelä RK, Pääkkö E, Suramo I, et al. Magnetic resonance imaging and magnetic resonance sialography of parotid glands in primary Sjögren's syndrome. Arthritis Rheum 2001;45:512-8. [Crossref] [PubMed]
- Milic VD, Petrovic RR, Boricic IV, et al. Major salivary gland sonography in Sjögren's syndrome: diagnostic value of a novel ultrasonography score (0-12) for parenchymal inhomogeneity. Scand J Rheumatol 2010;39:160-6. [Crossref] [PubMed]
- Kawamura H, Taniguchi N, Itoh K, et al. Salivary gland echography in patients with Sjogren’s syndrome. Arthritis Rheum 1990;33:505-10. [Crossref] [PubMed]
- Delli K, Arends S, van Nimwegen JF, et al. Ultrasound of the Major Salivary Glands is a Reliable Imaging Technique in Patients with Clinically Suspected Primary Sjögren's Syndrome. Ultraschall Med 2018;39:328-33. [Crossref] [PubMed]
- Zhang X, Zhang S, He J, et al. Ultrasonographic evaluation of major salivary glands in primary Sjögren’s syndrome: comparison of two scoring systems. Rheumatology (Oxford) 2015;54:1680-7. [Crossref] [PubMed]
- Mossel E, Delli K, van Nimwegen JF, et al. EULAR US-pSS Study Group. Ultrasonography of major salivary glands compared with parotid and labial gland biopsy and classification criteria in patients with clinically suspected primary Sjögren's syndrome. Ann Rheum Dis 2017;76:1883-9. [Crossref] [PubMed]
- Theander E, Mandl T. Primary Sjögren’s syndrome: diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system. Arthritis Care Res (Hoboken) 2014;66:1102-7. [Crossref] [PubMed]
- Qi X, Sun C, Tian Y, et al. Comparison of the diagnostic value of four scoring systems in primary Sjögren’s syndrome patients. Immunol Lett 2017;188:9-12. [Crossref] [PubMed]
- De Vita S, Lorenzon G, Rossi G, et al. Salivary gland echography in primary and secondary Sjogren’s syndrome. Clin Exp Rheumatol 1992;10:351-6. [PubMed]
- El Miedany YM, Ahmed I, Mourad HG, et al. Quantitative ultrasonography and magnetic resonance imaging of the parotid gland: can they replace the histopathologic studies in patients with Sjogren's syndrome? Joint Bone Spine 2004;71:29-38. [Crossref] [PubMed]
- Milic V, Petrovic R, Boricic I, et al. Ultrasonography of major salivary glands could be an alternative tool to sialoscintigraphy in the American-European classification criteria for primary Sjogren's syndrome. Rheumatology (Oxford) 2012;51:1081-5. [Crossref] [PubMed]
- Jousse-Joulin S, Devauchelle-Pensec V, Morvan J, et al. Ultrasound assessments of salivary glands in patients with primary Sjögren’s syndrome treated with rituximab: quantitative and Doppler wave analysis. Biologics 2007;1:311-9. [PubMed]
- Lee KA, Lee SH, Kim HR. Diagnostic and predictive evaluation using salivary gland ultrasonography in primary Sjögren's syndrome. Clin Exp Rheumatol 2018;36 Suppl 112:165-72. [PubMed]
- Astorri E, Sutcliffe N, Richards PS, et al. Ultrasound of the salivary glands is a strong predictor of labial gland biopsy histopathology in patients with sicca symptoms. J Oral Pathol Med 2016;45:450-4. [Crossref] [PubMed]
- Theander E, Vasaitis L, Baecklund E, et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjogren’s syndrome. Ann Rheum Dis 2011;70:1363.8.
- Martinoli C, Derchi LE, Solbiati L, et al. Color Doppler sonography of salivary glands. AJR Am J Roentgenol 1994;163:933-41. [Crossref] [PubMed]
- Salaffi F, Carotti M, Argalia G, et al. Usefulness of ultrasonography and color Doppler sonography in the diagnosis of major salivary gland diseases. Reumatismo 2006;58:138-56. [PubMed]
- Chikui T, Yonetsu K, Izumi M, et al. Abnormal blood flow to the submandibular glands of patients with Sjögren's syndrome: Doppler waveform analysis. J Rheumatol 2000;27:1222-8. [PubMed]
- Ralls PW, Mayekawa DS, Lee KP, et al. Color flow Doppler sonography in Graves disease: “thyroid inferno AJR Am J Roentgenol 1988;150:781-4. [Crossref] [PubMed]
- Carotti M, Salaffi F, Manganelli P, et al. Ultrasonography and colour doppler sonography of salivary glands in primary Sjögren's syndrome. Clin Rheumatol 2001;20:213-9. [Crossref] [PubMed]


