
Identifying the dissection plane for mastectomy—description and visualization of our technique
The quality and thickness of the mastectomy flaps is an important factor for an optimal outcome of an immediate breast reconstruction (1). However, this must never compromise oncologic safety and an adequate resection of the glandular tissue (2,3).
Freeman described the subcutaneous mastectomy and immediate breast reconstruction in 1962 (4). The technique is changing from always including the nipple-areola complex (NAC) to an acceptance of nipple sparing mastectomy in risk reducing mastectomies as well as in selected cancer patients. There is, however, an ongoing discussion about how much skin and subcutaneous tissue should be resected to perform an adequate oncological safe mastectomy, while still leaving viable skin flaps (5-11).
The optimal surgical plane provides the surgeon with a better overview of the operative field (12), resulting in adequate resection of breast parenchyma to obtain the best oncological outcome, while retaining the maximum amount of subcutaneous adipose tissue on the skin flaps to achieve the best aesthetic results (7,8,13,14). In this visualized surgery paper, we present our experience identifying the dissection plane using hydrodissection through an inframammary incision.
Surgical technique (Figure 1)

This video demonstrates a risk reducing case in which the dissection plane between the glandular tissue and the subcutaneous fatty tissue is well defined.
Preoperatively, the skin markings were made by a permanent marker with the patient in the standing position. The breast footprint and planned inframammary fold (IMF) incision was marked. We assessed the thickness and expected quality of the mastectomy flaps by T2-weighted magnetic resonance imaging (MRI) images of the breast just before surgery. Furthermore, the configuration of the glandular tissue behind the nipple areolar complex is evaluated. Some women have a narrow bundle of ducts with overlying rich subcutaneous flaps while others have wide bundles of ducts with thin overlying subcutaneous flaps.
Surgery commenced at the IMF by incision through the skin and subcutaneous adipose tissue at the level of the subcutaneous fascia of the breast obliquely and caudally to the muscle fascia. The glandular tissue was lifted by a retractor and the glandular tissue was then dissected from the thoracic wall and the pectoralis major muscle using monopolar cautery. The desired boundaries of cavity following the subglandular dissection was assessed by palpation.
The subcutaneous fascia of the breast bordering the subcutaneous fatty tissue and glandular tissue was identified and two Allis forceps were placed in the glandular tissue to pull the glandular tissue in a caudal direction whilst manually pushing the breast in a cranial direction.
Hydrodissection was used to assist in identifying the dissection plane. We infiltrate the entire breast by a blunt tip cannula using a solution of 1 L NaCl/1 mL epinephrine between the glandular tissue and the subcutaneous fatty tissue. Special focus is held on the retropapillar area where an extra amount is infused to lift the thin nipple areolar complex from the glandular tissue. The level of the infiltration is identified by vision at the incision line where the fascia separates the glandular tissue from the subcutaneous tissue. During infiltration the cannula is simply moved back and forth without force along the fascia. The dissection between the glandular and subcutaneous tissue is performed guided by vision using Metzenbaum scissors. The dissection consists of two movements; first, blunt dissection to separate the fat lobules from the glandular tissue between the Coopers ligaments, then the ligaments are cut by a sliding movement towards the top of the ligaments to release these from their attachment towards the dermis (Figure 2). The cut has to be as close to the skin as possible in order to remove possible glandular tissue within the ligaments. The subcutaneous dissection is first performed inferiomedially and inferolaterally to the level of the NAC. The subcutaneous fascia/dissection plane merges towards the skin as the nipple is approached. The nipple is release by sharp dissection through the glandular ducts. The location of the ducts is marked by 4.0 nylon in the glandular specimen for pathologic evaluation. The superomedial and superolateral part of the breast is dissected. The superolateral part of the dissection can be somewhat challenging, however in some cases it is possible to pull the entire breast specimen out thought the IMF incision for better visualization of the most cranial boundary of the breast attachment towards the axilla.
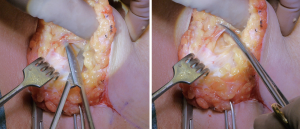
The breast specimen can then be assessed. The optimal specimen reveals the smooth surface of the fascia without any fatty tissue. There is always some fatty tissue left, but this should be kept to a minimum. The mastectomy flap thickness and quality can then be assessed prior to reconstruction.
Surgical technique (Figure 3)

This video demonstrates a tumor case in which the dissection plane between the glandular tissue and the subcutaneous fatty tissue is less defined.
The video shows the same technique as Figure 1, however, the patient had a tumor inferior to the NAC and the dissection was more superficial in this area as demonstrated by the surgical technique as well as the breast specimen where a layer of fatty tissue is preserved over the tumor site in order to secure cancer free resection margins.
Comments
In this visualized surgery paper, we demonstrate our technique for identifying the mastectomy dissection plane when performing mastectomy prior to immediate breast reconstruction. We use preoperative MRI aiming to assess the thickness of the skin flaps and thus get an idea about the location and nature of the dissection plane, although it seems that a well-defined dissection plane cannot be expected to be present in all patients as described in a histological evaluation of breast specimens by Beer et al. (7). However, this is debatable as Larsen revealed a consistent and distinct layer of non-breast-bearing subcutaneous tissue with a median thickness of about 1 cm between the dermis and breast parenchyma (11). However, although the median thickness of this subcutaneous layer may be 1 cm, it is variable, an unpredictable and a universal thickness cannot be used as described by Robertson et al. (9). Furthermore, Torresan et al. found a high prevalence of glandular breast tissue in skin flaps thicker than 5 mm (8). Our main focus of the mastectomy is to remove the breast parenchyma adequately preserving the subcutaneous fatty tissue on the mastectomy skin flaps. We are continuously guided by pathology and rarely have to make any re-resections. However, no matter how we perform the mastectomy, modified or total mastectomy, we will leave glandular tissue behind as described. The two techniques, however, seems to be equally effective according to Barton et al. (17). Even though we know that we are performing the dissection in the right plane, glandular tissue will occur outside the dissection plane according to Karusseit et al. (10) and Griepsma et al. They showed that the residual breast tissue was predominantly left in the superficial plane in the center of the breast and the lower outer quadrant (18). We use hydrodissection as an aide to identify the dissection plane. We infiltrate the tissue carefully and do not “over-infiltrate” as we believe that this may damage the blood supply to the skin flaps. However, we find that hydrodissection enable us to better identify the correct dissection plane as we perform the dissection guided by vision. The fascia is easier to identify and vasoconstriction minimizes bleeding. We have earlier published papers in which we have demonstrated that it is possible to safely perform hydrodissection in terms of flap survival without visual guidance using palpation instead (19-21), however there is a learning curve to using this technique and we recommend using the technique illustrated in this paper. Hydrodissection can be used to facilitate NAC dissection as demonstrated by Folli et al. (22). However, Chun et al. found that tumescent mastectomy technique was associated with a substantial increase in the risk for skin flap necrosis (23). However, in our experience this is not the case.
When we perform the IMF dissection, we aim to follow the subcutaneous fascia caudally, however the definition of the subcutaneous fascia seems variable. This experience is supported by the findings by Muntan et al. who found no demonstrable ligamentous structure of dense regular connective tissue in the fold region in 12 specimens (24), although van Straalen et al. found a transversely oriented inframammary ligament in all of their specimens (25). The interest in a well-defined IMF probably arises from a reconstructive perspective as a well-defined lower border enables the reconstructive surgeon to achieve a better reconstructive result. Two decades ago Carlson et al. raised the question “If the tissue below the IMF should be included in the breast specimen as only a minimal amount of breast tissue was left behind and did not appreciably effect the completeness of a mastectomy?” (26). We believe that all glandular tissue should be removed, when possible. We are constantly trying to optimize and balance oncologic safety with reconstructive options by refining our surgical techniques. However, the outcome of our efforts can be difficult to measure with regard to oncologic safety as adjuvant treatment, fortunately, is constantly developing and improving.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Gunnarsson GL, Heidemann LN, Bille C, et al. Nipple sparing mastectomy and the evolving direct to implant breast reconstruction. Gland Surg 2018;7:267-72. [Crossref] [PubMed]
- Adam H, Bygdeson M, de Boniface J. The oncological safety of nipple-sparing mastectomy - a Swedish matched cohort study. Eur J Surg Oncol 2014;40:1209-15. [Crossref] [PubMed]
- Romics L Jr, Stallard S, Weiler-Mithoff E. Oncologic safety of skin-sparing mastectomy followed by immediate breast reconstruction: rate and localization of recurrences, and impact of reconstruction techniques. Orv Hetil 2013;154:163-71. [Crossref] [PubMed]
- Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull 1962;30:676-82. [Crossref] [PubMed]
- Toth BA, Forley BG, Calabria R. Retrospective study of the skin-sparing mastectomy in breast reconstruction. Plast Reconstr Surg 1999;104:77-84. [Crossref] [PubMed]
- Ashikari AY, Kelemen PR, Tastan B, et al. Nipple sparing mastectomy techniques: a literature review and an inframammary technique. Gland Surg 2018;7:273-87. [Crossref] [PubMed]
- Beer GM, Varga Z, Budi S, et al. Incidence of the superficial fascia and its relevance in skin-sparing mastectomy. Cancer 2002;94:1619-25. [Crossref] [PubMed]
- Torresan RZ, dos Santos CC, Okamura H, et al. Evaluation of residual glandular tissue after skin-sparing mastectomies. Ann Surg Oncol 2005;12:1037-44. [Crossref] [PubMed]
- Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg 2014;101:899-911. [Crossref] [PubMed]
- Karusseit VO, Oberholzer HM, Irsigler NG, et al. Determination of the accuracy of juxtacapsular dissection of the breast. What is left behind? Int J Surg 2014;12:384-9. [Crossref] [PubMed]
- Larson DL, Basir Z, Bruce T. Is oncologic safety compatible with a predictably viable mastectomy skin flap? Plast Reconstr Surg 2011;127:27-33. [Crossref] [PubMed]
- Galimberti V, Vicini E, Corso G, et al. Nipple-sparing and skin-sparing mastectomy: Review of aims, oncological safety and contraindications. Breast 2017;34 Suppl 1:S82-4. [Crossref] [PubMed]
- Newman LA, Kuerer HM, Hunt KK, et al. Presentation, treatment, and outcome of local recurrence afterskin-sparing mastectomy and immediate breast reconstruction. Ann Surg Oncol 1998;5:620-6. [Crossref] [PubMed]
- Simmons RM, Fish SK, Gayle L, et al. Local and distant recurrence rates in skin-sparing mastectomies compared with non-skin-sparing mastectomies. Ann Surg Oncol 1999;6:676-81. [Crossref] [PubMed]
- Bille C, Dalaei F, Thomsen JB. Identifying the dissection plane for mastectomy—description and visualization of our technique: video 1. Asvide 2019;6:296. Available online: http://www.asvide.com/watch/32981
- Bille C, Dalaei F, Thomsen JB. Identifying the dissection plane for mastectomy—description and visualization of our technique: video 2. Asvide 2019;6:297. Available online: http://www.asvide.com/watch/32982
- Barton FE Jr, English JM, Kingsley WB, et al. Glandular excision in total glandular mastectomy and modified radical mastectomy: a comparison. Plast Reconstr Surg 1991;88:389-92; discussion 393-4. [Crossref] [PubMed]
- Griepsma M, de Roy van Zuidewijn DB, Grond AJ, et al. Residual breast tissue after mastectomy: how often and where is it located? Ann Surg Oncol 2014;21:1260-6. [Crossref] [PubMed]
- Gunnarsson GL, Thomsen JB. Prepectoral Hammock and Direct-to-implant Breast Reconstruction in 10 Minutes: A Focus on Technique. Plast Reconstr Surg Glob Open 2018;6:e1931. [Crossref] [PubMed]
- Gunnarsson GL, Bille C, Reitsma LC, et al. Prophylactic Nipple-Sparing Mastectomy and Direct-to-Implant Reconstruction of the Large and Ptotic Breast: Is Preshaping of the Challenging Breast a Key to Success? Plast Reconstr Surg 2017;140:449-54. [Crossref] [PubMed]
- Gunnarsson GL, Borsen-Koch M, Wamberg P, et al. How to perform a NAC sparing mastectomy using an ADM and an implant. Gland Surg 2014;3:252-7. [PubMed]
- Folli S, Curcio A, Buggi F, et al. Improved sub-areolar breast tissue removal in nipple-sparing mastectomy using hydrodissection. Breast 2012;21:190-3. [Crossref] [PubMed]
- Chun YS, Verma K, Rosen H, et al. Use of tumescent mastectomy technique as a risk factor for native breast skin flap necrosis following immediate breast reconstruction. Am J Surg 2011;201:160-5. [Crossref] [PubMed]
- Muntan CD, Sundine MJ, Rink RD, et al. Inframammary fold: a histologic reappraisal. Plast Reconstr Surg 2000;105:549-56; discussion 557. [Crossref] [PubMed]
- van Straalen WR, Hage JJ, Bloemena E. The inframammary ligament: myth or reality? Ann Plast Surg 1995;35:237-41. [Crossref] [PubMed]
- Carlson GW, Grossl N, Lewis MM, et al. Preservation of the inframammary fold: what are we leaving behind? Plast Reconstr Surg 1996;98:447-50. [Crossref] [PubMed]


