Oncoplastic options in breast conservative surgery
Conservative surgery has become the elective alternative in the treatment of breast cancer, However, to achieve tumor-free margins and to reduce the risk of local recurrence, in case of large lesions, small breasts, or more than 30% of breast volume resection, the procedure can often compromise the aesthetic result. To overpass this situations, different surgical procedures, called oncoplastic techniques have been described to optimize the efficacy of conservative surgery, both in terms of local control and cosmetic results. Indications, advantages, and limitations of different oncoplastic approaches, and their results are discussed.
Surgical treatment of breast cancer has been modified during the past decades. The long-term results of several studies conducted worldwide have definitely confirmed that conservative surgery (CS) and radical mastectomy have similar survival rates, endorsing the CS as the gold standard of therapy for most women with breast cancer (1,2).
The long-term success of the CS can be measured by two variables:
- Local control rate;
- Cosmetic outcome of the conserved breast.
Sometimes, in CS it can be difficult for the surgeon to adequately meet these two points, especially when trying to resect large lesions or in small breasts.
The extent of parenchymal removal and the skin resection is directly correlated with the cosmetic result: the higher volumes of tissue are removed, the risk of a poor outcome cosmetic increases. Olivotto et al. (3) and Mills et al. (4) have reported that the cleavage of a volume greater than 70 cm3 parenchyma in medium sized breasts often leads to unsatisfactory aesthetic results. The Rochefordiere et al. (5) and Taylor et al. (6) have documented a lower cosmetic outcome in patients who had a volume of removed tissue greater than 86 and 100 cm3 respectively.
Cochrane et al. (7) demonstrated that the cosmetic result is impaired when the weight of the piece: breast volume ratio is greater than 10%.
This unfavorable correlation explains why some surgeons have favored more limited resections, describing techniques such as lumpectomy (primary tumor excision with margins of healthy breast tissue less than 1 cm), as opposed to classical proposed quadrantectomy Veronesi et al. (8) (“a large quadrant resection of primary carcinoma house with at least 2 cm of healthy tissue surrounding the tumor and including removal block a large portion of the overlying skin en bloc to the pectoralis major muscle fascia”).
The magnitude of parenchymal excision is also directly correlated with the rate of local control of cancer. Therefore, with use of more limited resections, results in an increased risk of local relapse. Many studies have confirmed this hypothesis. In Phase II Trial Milano 1,705 patients with tumors up to 2.5 cm in diameter were randomly selected to receive (I) lumpectomy (excision near the tumor) or (II) quadrantectomy (excision of tumors with macroscopically apparent margins 2 cm) including the skin and pectoral fascia.
Although the overall survival rate was not different in the two groups, the local recurrence rate at 5 years was much higher in the lumpectomy group (7.0% vs.2.2%).
Holland et al. demonstated that the risk of leaving the engaged margins operated breast was inversely related to the degree of local control of the disease. Resecting the tumor healthy tissue around 1 cm range, the likelihood of residual cancer foci was about 59% whereas with 3 cm removed, decreases to 17% (9).
Technological advances in diagnosis, mammography and MRI, as well as greater use oy punctures preoperative neoadjuvant systemic treatments have expanded the indications, arriving today in Argentina usage rates of this procedure to 70-80% of patients with breast cancer. However, in USA is below 50% (10) and 58% in Italy (11). Among the factors that may explain this under CC are the concerns of the patient and the surgeon for control of the disease in terms of local recurrence or poor outcome.
In an attempt to optimize the balance between the risk of local recurrence and cosmetic results in DC, new surgical procedures that combine the principles of surgical oncology and plastic surgery have been introduced in recent years (12-15). These new techniques, called “oncoplastic” may allow resection of a greater amount of breast tissue and safer margins without compromising the aesthétic result.
Oncoplastic procedures are technically more demanding, requiring training and planning, and sometimes more time consuming.
These procedures are usually done in one surgical time, and the patient leaves the operating room with minimum asymmetry or deformity.
When designing an oncoplástic procedure, steps must be met: careful planning of skin incisions, parenchymal resections in block up to the pectoral fascia, metal clip placement in the resection margins, proper gland remodeling after parenchymal resection, repositioning of the nipple-areola complex (CAP) in the center of the breast, and the correction of the contralateral breast for better symmetry.
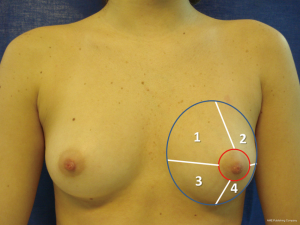
Depending on the location of the lesion in the breast (Figure 1), different oncoplastic techniques can be used (16,17).
Quadrantectomy with round block or Benelli technique
This oncoplastic procedure has its best application in periareolar lesions treatment in the upper quadrants, specially, in breasts, with moderate ptosis or hypertrophy. It is based on the mammary modelling technique described by Louis Benelli.
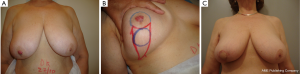
In this technique, two concentric rings of different diameters are marked and designed around the nipple areolar complex. It allows resect, repair, model and lift the NAC. The skin between the two circles is excised (Figure 2A,B,C,D). This incision allows convenient access to the region through a periareolar incision, which is wider compared with traditional conservative techniques.
Ideal for:
- Ptotic breasts, large or medium size;
- Raising the NAC;
- Reducing the areola;
- Breast modeling;
- You can flatten the breast (advantage or disadvantage);
- Superior Quadrants tumors resection around areola.
The remodeling of the breast is performed with the residual gland, dissecting above the pectoralis major muscle with the use of electrocautery. Care must be taken in dissecting major vascular pedicles perforators’ vessels between pectoral muscle and the preserved breast, to minimize the risk of NAC ischemia, residual glandular tissue necrosis and to minimize the risk of hematoma. The larger circle diameter is reduced by a circular suture around the new areolar margin.
Axillary dissection is usually performed through a separate incision, but on rare occasions may be performed through the same periareolar incision. If the two are concentric circles, the NAC is not elevated. If the outer circle is centered around a point above the existing NAC, this may be rise, and a little pseudoptosis can be simultaneously corrected. Regarding the diameter of the inner and outer part of the oval design, the later must not exceed that of the existing areola diameter of 20-25 mm more toward lateral or medial, making an oval, to prevent distention of the circumareolar scar or excessive flattening of the breast.
Central quadrantectomy with a skin-glandular flap or Grisotti technique
This technique is used in oncoplástic designs for subareolar lesions and Paget’s disease. These tumors often tend to be excluded from conservative surgery techniques due to oncologic concerns about multicentricity or multifocality association, and were treated with mastectomy due to bad cosmetic result, associated with NAC amputation.
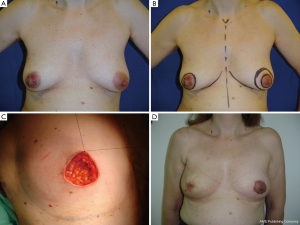
This simple technique allows conservative treatment for retroareolar tumors or in Paget disease, with oncologic safety and excellent aesthetic results (Figure 3A,B,C). Resection is performed including a NAC cylinder and the parenchyma up to the pectoral fascia. The creation of a new NAC is achieved by a dermo-glandular flap, mobilized from residual gland in the lower breast pole. The flap is deepithelized, except a circular area of skin near the defect which will replace the NAC resected area by rotation.
The flap is incised medially, up to the pectoral fascia. It is very important to accurately separate the fascia flap to allow better rotation and advancement. The flap is mobilized and sutured to the gland superiorly in order to provide adequate projection and prevent dead spaces. If desired by the patient, the nipple can be reconstructed at a later stage. Consideration should be given to flap vascularization, in order to minimize the risk of ischemic injury.
Therapeutic reductions
This oncoplastic techniques can be used for tumors located in the upper or lower quadrants in the periareolar region, and are particularly indicated in patients with macromastia. This therapeutic reductions can be based on V design (Figure 4A,B,C) or over a wise keyhole inverted T pattern. The areola can be moved as necessary to change the position of the NAC, and the lesion is included within the resection area (Figure 5A,B,C,D,E).
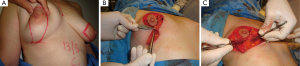
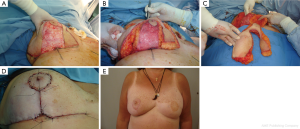
For tumors located in inferomedial or inferolateral quadrants, the keyhole pattern may rotate slightly and allows lateral or medial excision. NAC is mobilized in the opposite direction of the surgical defect, leaving an inverted T scar.
Using techniques of reduction mammoplasty, tumors can be resected easily with large safety margins, even in small breasts, avoiding major cosmetic defects. These techniques can also facilitate the completion of radiation therapy in the postoperative period, particularly in women with macromastia (Figure 6A,B,C,D,E).
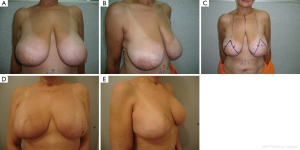
Reducing the size of the breast by mastoplasty techniques, significantly reduces the risk of retraction, without affecting adjuvant therapies or clinical and radiological follow-up (18,19).
The resection should be full thickness and glandular tissue must be advanced to close the defect (20).
While performing symmetrization procedures in the contralateral breast, the surgeon should take the opportunity to remove any suspicious tissue that may have been revealed by a preoperative mammogram. Oncoplastic surgery techniques can expand the indications for CS, but since oncoplastic techniques have been introduced recently, little data are available to measure results (21).
In a prospective study to evaluate cosmetic and oncologic results after performing oncoplastic techniques, Clough et al. (22) collected data from 101 patients with breast cancer with a median size of 32 mm. The most common surgical procedure was breast reduction with keyhole pattern (83% of cases). The average weight of the resected specimen after oncoplastic procedures was significantly higher (220 g) compared with the average weight of a lumpectomy specimen in the same institution (40 g). After a follow-up of 3.8 years, the rate of complications after oncoplastic surgery (fat necrosis, fibrosis and hypertrophic scarring) was 10% and the cosmetic result was acceptable (excellent, good or fair) in 88% of cases. The local recurrence rate at 5 years was 9.4% and the overall survival rate was 82.8%, which compares favorably with most CS studies (22).
In a recent study, we prospectively studied 30 consecutive patients with breast cancer undergoing oncoplastic procedures (group 1) and 30 patients undergoing traditional lumpectomy (group 2).
Oncologic evaluated stage, surgical procedures, the volume of breast tissue removed, and histopathology of the tumors, with specific details on the surgical margins. Patients in group 1 were younger than patients who had a classic lumpectomy.
Oncoplastic approach allowed large resections, with an average volume of 200 cm3 sample, compared with 117 cm3 in the quadrantectomy group. Surgical margins were negative in 25 of the 30 cases (83%) in group 1, and 17 of 30 cases (56%) in group 2, the average length of the surgical wound was 8.5 mm in group 1 and 6.5 mm in group 2, although the difference was not statistically significant (23).
As Masetti et al. observed several studies and world experience suggest that oncoplastic techniques can optimize cancer treatment with oncological safety and good cosmetic results in CS.
Surgeons with interest in the surgical treatment of breast cancer, should seek appropriate training in oncoplastic surgery in order to offer these procedures to their patients (24).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [PubMed]
- Olivotto IA, Rose MA, Osteen RT, et al. Late cosmetic outcome after conservative surgery and radiotherapy: analysis of causes of cosmetic failure. Int J Radiat Oncol Biol Phys 1989;17:747-53. [PubMed]
- Mills JM, Schultz DJ, Solin LJ. Preservation of cosmesis with low complication risk after conservative surgery and radiotherapy for ductal carcinoma in situ of the breast. Int J Radiat Oncol Biol Phys 1997;39:637-41. [PubMed]
- de la Rochefordière A, Abner AL, Silver B, et al. Are cosmetic results following conservative surgery and radiation therapy for early breast cancer dependent on technique? Int J Radiat Oncol Biol Phys 1992;23:925-31. [PubMed]
- Taylor ME, Perez CA, Halverson KJ, et al. Factors influencing cosmetic results after conservation therapy for breast cancer. Int J Radiat Oncol Biol Phys 1995;31:753-64. [PubMed]
- Cochrane RA, Valasiadou P, Wilson ARM, et al. Cosmesis and satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised. Br J Surg 2003;90:1505-9. [PubMed]
- Veronesi U, Volterrani F, Luini A, et al. Quadrantectomy versus lumpectomy for small size breast cancer. Eur J Cancer 1990;26:671-73. [PubMed]
- Holland R, Veling SH, Mravunac M, et al. Histologic multifocality of Tis, T1-2 breast carcinoma. Implications for clinical trials of breast-conserving surgery. Cancer 1985;56:979-90. [PubMed]
- Morrow M, White J, Moughan J, et al. Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J Clin Oncol 2001;19:2254-62. [PubMed]
- Pellegrini L. SDO ASSR-2002, Italian Ministry of Health, Rome, 2002. (oral communication)
- Baildam AD. Oncoplastic surgery of the breast. Br J Surg 2002;89:532-33. [PubMed]
- Asgeirsson KS, Rasheed T, McCulley SJ, et al. Oncological and cosmetic outcomes of oncoplastic breast conserving surgery. Eur J Surg Oncol 2005;31:817-23. [PubMed]
- Quinn McGlothin TD. Breast surgery as a specialized practice. Am J Surg 2005;190:264-68. [PubMed]
- Brédart A, Petit JY. Partial mastectomy: a balance between oncology and aesthetics? Lancet Oncol 2005;6:130. [PubMed]
- Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol 2005;6:145-57. [PubMed]
- Masetti R, Pirulli PG, Magno S, et al. Oncoplastic techniques in the conservative surgical treatment of breast cancer. Breast Cancer 2000;7:276-80. [PubMed]
- Smith ML, Evans GR, Gurlek A, et al. Reduction mammaplasty: its role in breast conservation surgery for early-stage breast cancer. Ann Plast Surg 1998;41:234-39. [PubMed]
- Cody HS 3rd. Current surgical management of breast cancer. Curr Opin Obstet Gynecol 2002;14:45-52. [PubMed]
- Clough KB, Thomas SS, Fitoussi AD, et al. Reconstruction after conservative treatment for breast cancer: cosmetic sequelae classification revisited. Plast Reconstr Surg 2004;114:1743-53. [PubMed]
- Clark J, Rosenman J, Cance W, et al. Graham Extending the Indications for breast-conserving treatment to patients with locally advanced breast cancer. Int J Radiat Oncol Biol Phys 1998;42:345-50. [PubMed]
- Clough KB, Lewis JS, Couturaud B, et al. Oncoplastic techniques allow resection for breast-conserving therapy of breast carcinomas. Ann Surg 2003;237:26-34. [PubMed]
- Kaur N, Petit JY, Rietjens M, et al. Comparative study ofsurgical margins in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Onco 2005;12:539-45.
- Masetti R, Di Leone A, Franceschini G, et al. Oncoplastic techniques in the conservative surgical treatment of breast cancer: an overview. Breast J 2006;12:S174-80. [PubMed]