Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases: commentary on the IBCSG 23-01 Trial
Minimally invasive and targeted breast cancer surgery is the focus of great current interest. Over the last several decades there has been a strong trend toward minimizing the extent of surgical resection in the management of patients with newly diagnosed breast cancer. Initially the focus was a shift from mastectomy to breast conservation. More recently, decreasing the extent of axillary surgery, and targeting more extensive operation to patients most likely to benefit in terms of local control and improved outcomes, is the topic of great discussion. Breast surgical oncologists have rapidly and successfully transitioned from the routine use of axillary lymph node dissection (ALND) to sentinel lymph node (SLN) biopsy for staging the axilla in clinically node negative patients. This approach limits the use of ALND to those patients with pathologically-proven axillary lymph node metastases.
The current highly debated question is whether or not all SLN-positive patients actually require a completion ALND. Analysis of data from the National Cancer Data Base (NCDB) showed that there has been a trend towards omitting ALND in patients with low volume axillary disease. The NCDB report revealed that the percentage of patients undergoing SLN biopsy alone (without completion ALND) with microscopic nodal disease increased from 24.7% in 1998 to 45.3% in 2005 (1). Similarly, analysis of the Surveillance, Epidemiology and Survival Registry (SEER) data also has shown that the proportion of women undergoing SLN biopsy only for microscopic nodal disease increased from 21.1% in 1998 to 37.6% in 2004 (2). Thus even prior to presentation of the results of the American College of Surgeons Oncology Group (ACOSOG) Z0011 study (3,4) or the current publication of the results of the IBCSG 23-01 study (5), completion ALND was being performed less frequently for selected patients with nodal micrometastases.
Shortly after the rapid adoption of SLN biopsy for staging the axilla in clinically node-negative breast cancer patients, came publication of the first reports on patients with positive SLNs in whom ALND was omitted. These retrospective studies evaluating SLN-positive patients who did not undergo axillary clearance suggested that axillary recurrence rates are low, but higher than after ALND. In a 2004 report, 210 SLN-positive patients, of whom 68.6% had nodal disease detected only by immunohistochemistry and who did not undergo ALND, were studied. For those patients treated with SLN biopsy only, the axillary recurrence rate was 1.4% after a median follow-up of 2.5 years, which was significantly greater than the 0.2% rate seen in 1,132 SLN-positive patients who did undergo completion ALND during the same time period (6). Another retrospective study evaluated 81 patients with isolated tumor cells (ITCs) only in a SLN and found that of 31 patients who did undergo ALND, 12.9% had additional positive lymph nodes. After 3 years of follow up, no axillary recurrences were seen in the patients treated by ALND nor in the 50 patients who underwent SLN biopsy alone (7).
The International Breast Cancer Study Group (IBCSG) trial 23-01 was activated in April 2001 and accrued patients through February 2010, randomizing those patients with micrometastasis or ITCs in the SLN, based on hematoxylin and eosin pathology evaluation, to ALND or no ALND. The IBCSG study (like ACOSOG Z0011) was a randomized, phase III trial to assess the impact of avoiding ALND on long-term patient outcomes and enrolled patients during a time frame in which surgeons were already adapting their practices and decreasing their performance of ALND for micrometastatic SLN disease.
In many ways, the IBCSG 23-01 results are very similar to the findings from the ACOSOG Z0011 study. Both of these studies were evaluated as non-inferiority trials which randomized patients with positive SLNs to ALND versus no ALND, and both studies showed no significant difference in five-year disease-free survival or locoregional recurrence rates between the two groups. Similar to the ACOSOG Z0011 study, the IBCSG 23-01 study also closed early after meeting less than 50% of its targeted accrual goal. Both of these studies had both lower accrual rates and lower event rates than had been predicted at the time of study design.
The IBCSG 23-01 study limited enrollment to patients with micrometastases or ITCs in the sentinel lymph nodes, different from the Z0011 eligibility criteria which allowed micrometastatic or macrometastatic disease and in which 50% of patients (430/856) had macrometastatic disease, 35% (301/856) had micrometastases and the remainder no or unknown extent of nodal disease. Additionally, in the IBCSG study, 95% of patients had only one positive SLN whereas in the Z0011 study 79% (324/415) of the SLN only group had zero or one positive SLN. Thus, nodal burden was lower in the IBCSG 23-01 study than ACOSOG Z0011 (Table 1). Therefore, in the context of Z0011, the results of the IBCSG 23-01 study, in which 95.6% of patients had only one positive SLN limited to ITCs or micrometastatic disease, are not surprising. Namely, local control and disease-free survival were not different with or without ALND after a median follow-up of 5 years in this highly selected group of patients. The study provides additional data to confirm that for this limited group of patients, mainly those with small ER-positive tumors with low nodal disease burden undergoing breast conservation with radiation, ALND might be avoided safely. It is important to note that in both of these studies, more than 90% of patients received systemic therapy.
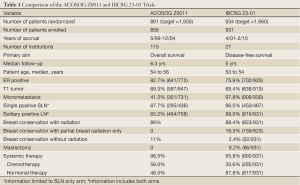
Full Table
So what does IBCSG 23-01 add beyond what we have learnt from Z0011? One of the important differences between the two studies is that the IBCSG 23-01 study did allow enrollment of patients undergoing mastectomy and therefore it opens the discussion for consideration of avoiding ALND for those patients with a positive SLN at the time of mastectomy. Unfortunately, however, only 86 patients enrolled in IBCSG 23-01underwent mastectomy, accounting for just 9% of each arm. Therefore this number (42 patients without ALND) is really too small to allow any extrapolation of the trial results to patients that did not undergo breast conservation surgery and radiation therapy. Further evaluation of this question of avoiding ALND for positive SLN in patients undergoing mastectomy is required before adopting this into routine clinical practice.
Another interesting aspect of the IBCSG 23-01 study is that 19% of patients received partial breast radiotherapy in the form of intraoperative radiation. But again this subset of patients is too small (80 patients with SLN only) and the follow-up too short to draw meaningful conclusions about the suitability of omission of ALND for patients with nodal micrometastases or ITCs undergoing adjuvant partial versus whole breast radiation.
There has been much debate about the proportion of newly diagnosed breast cancer patients who would benefit from the findings of the ACOSOG Z0011 and IBCSG 23-01 studies. In the IBCSG 23-01 study, the trialists report that 6,681 patients were registered before surgery of which 934 patients were randomized, indicating that only 14% of eligible breast cancer patients met the inclusion criteria for this study and underwent randomization. It is anticipated that this may be due to both node negative patients and also patients with multiple positive nodes and other factors, however the breakdown is unknown. Other reports also have shown that many patients evaluated within a breast center may not meet the defined eligibility criteria for avoidance of ALND in the presence of a positive SLN (8-10). Reasons may include tumor size, tumor biology, identification of extensive nodal disease at operation, patients being identified as pathologically node positive on fine needle aspiration prior to surgery, triage to neoadjuvant chemotherapy, selection of mastectomy, or a desire to avoid adjuvant breast radiation after breast conserving surgery.
The use of axillary ultrasound may help define the group of patients most likely to meet criteria for avoidance of a completion ALND in the face of a positive SLN. Axillary ultrasound can be a tool to identify preoperatively those patients with higher nodal burden and, in the setting of a negative preoperative axillary ultrasound and positive SLN, rates of high nodal disease burden are low providing further information to help the clinician feel comfortable with potential omission of ALND in appropriate patients. For newly diagnosed invasive breast cancer patients with a clinically negative axilla, and selected for primary surgical treatment without neoadjuvant chemotherapy, the finding of sonographically normal axillary lymph nodes was associated with a positive SLN in only one-fifth of patients, of whom only 4% had more than 2 positive nodes, with a median metastasis size of 2.8 mm (11).
While the IBCSG 23-01 data supports omission of ALND for the select group of patients with small, ER+ tumors undergoing breast conservation with planned whole breast radiation, it suggests that there is interest in further investigation of the effect of omitting ALND in SLN-positive mastectomy patients and patients undergoing partial breast irradiation. If the primary benefit to these patients is systemic adjuvant therapy and not locoregional therapy, based on favorable tumor biology, this would seem like a logical next step.
However, data from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial which evaluated the suitability of SLN biopsy alone for SLN-negative clinically node-negative patients, by comparing SLN negative patients undergoing SLN biopsy alone to those undergoing SLN biopsy followed by ALND, suggests there is a significant survival disadvantage, after a median 8 years of follow-up, for women with occult nodal disease (12). In this study, 15.9% of 3,887 patients who were SLN negative by conventional histology had their SLNs reevaluated with immunohistochemistry by a central pathology laboratory and were found to be node-positive. Only 0.4% of patients had macrometastatic disease, while 4.4% had micrometastases and 11.1% ITCs. In the NSABP B-32 study, more than 85% of patients received adjuvant systemic therapy. Survival estimates by the presence or absence of occult immunohistochemically-detected SLN metastasis in hematoxylin and eosin-negative SLNs were all statistically significant. The 5-year overall survival was 94.6% versus 95.8%, the 5-year disease-free survival was 86.4% versus 89.2% and the 5-year distant disease-free survival was 89.7% versus 92.5%, respectively, all P<0.05. The 8-year median follow-up in the B-32 study is longer that reported for Z0011 and IBCSG 23-01. This would caution us to be clear in discussion with patients, especially those who are younger and have otherwise favorable-prognosis tumors, that the long-term outcomes of SLN biopsy alone for low volume axillary disease are unknown.
The IBCSG 23-01 adds further data that suggests that SLN biopsy only may be adequate surgical treatment for micrometastatic disease identified in a solitary SLN in women with small ER-positive tumors undergoing breast-conserving surgery followed by whole breast adjuvant radiotherapy. While this study doesn’t appear to add much beyond ACOSOG Z0011, it does invite us to look at those patients undergoing partial breast irradiation and those undergoing mastectomy, as potential candidates for limited axillary surgery as well. It may be that SLN biopsy is a form of super-selective therapeutic ALND for some types of breast cancer in patients with low volume axillary disease. Further prospective clinical trials are required to address these important patient populations.
ALND still has a place in the management of breast cancer patients. Ultimately the balance is between side effects of the ALND and oncologic control. Although the benefit of ALND has been debated for years, adequate oncologic surgery for cancer control continues to have a place in the modern multidisciplinary management of breast cancer. While IBCSG 23-01 and similar studies have intriguing 5-year outcome data, long-term follow-up data may not bear out these relatively early results as seen with late outcome analysis and meta-analysis of other breast cancer trials data (13,14). As surgeons it is also important that we realize that although it may be safe to avoid ALND in the ideal setting in which both adjuvant radiation and adjuvant systemic therapy are given, in reality not all patients do or plan to complete all the recommended adjuvant therapy, including oral therapies such as tamoxifen, due to the perceived or actual side effects of these treatments. Further work is needed to improve our understanding of breast tumor biology in order to identify those patients for whom less extensive surgery will not compromise long-term oncologic outcomes. In the meantime, patient counseling of the options for management of low volume axillary disease should address exactly what data we currently do have and what remains, as yet, unknown.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol 2009;27:2946-53. [PubMed]
- Yi M, Giordano SH, Meric-Bernstam F, et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol 2010;17 Suppl 3:343-51. [PubMed]
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. [PubMed]
- Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 2010;252:426-32; discussion 432-3. [PubMed]
- Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 2013;14:297-305. [PubMed]
- Naik AM, Fey J, Gemignani M, et al. The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection: a follow-up study of 4008 procedures. Ann Surg 2004;240:462-8; discussion 468-71. [PubMed]
- Degnim AC, Zakaria S, Boughey JC, et al. Axillary recurrence in breast cancer patients with isolated tumor cells in the sentinel lymph node Ann Surg Oncol 2010;17:2685-9. [PubMed]
- Ainsworth RK, Kollias J, Blanc AL, et al. The clinical impact of the American College of Surgeons Oncology Group Z-0011 trial - Results from the BreastSurgANZ National Breast Cancer Audit. Breast 2013. [Epub ahead of print]. [PubMed]
- Delpech Y, Bricou A, Lousquy R, et al. The Exportability of the ACOSOG Z0011 Criteria for Omitting Axillary Lymph Node Dissection After Positive Sentinel Lymph Node Biopsy Findings: A Multicenter Study. Ann Surg Oncol 2013;20:2556-61. [PubMed]
- Yi M, Kuerer HM, Mittendorf EA, et al. Impact of the American College of Surgeons Oncology Group Z0011 criteria applied to a contemporary patient population. J Am Coll Surg 2013;216:105-13. [PubMed]
- Hieken TJ, Boughey JC, Jones KN, et al. Preoperative axillary imaging with percutaneous lymph node biopsy is valuable in the contemporary management of breast cancer patients. Surgery 2013.
- Weaver DL, Ashikaga T, Krag DN, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med 2011;364:412-21. [PubMed]
- Fentiman IS. Long-term follow-up of the first breast conservation trial: Guy’ wide excision study. Breast 2000;9:5-8. [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [PubMed]


