
The breast surgeons’ approach to mastectomy and prepectoral breast reconstruction
Good planning for any oncologic surgery, starts with an understanding of the disease for which surgery is being performed along with a clear definition of the goal of the procedure. Regardless of the specific surgical procedure, for the treatment of breast cancer, these principles mark the foundation of our approach to every patient, every time.
There were an estimated 252,710 new cases of breast cancer in 2017 (1), with 40,610 deaths, translating to 1 death for every 37 women diagnosed (2). In the United States, as of January 2018, there were more than 3.4 million women either with a history of breast cancer, or being actively treated for the disease (3). Not including benign breast biopsies and cosmetic breast surgery, there are over half a million-breast cancer related surgeries performed per year in the United States (4). The fact that that breast cancer survival after both breast conserving surgery (BCS) and mastectomy is based on stage at diagnosis (tumor size and characteristics, lymph node status, and presence of systemic disease) and not the type surgery performed, is frequently overlooked. Regardless of the surgical approach, the oncologic principles of complete tumor removal, appropriate lymph node assessment and patient selection remain paramount in achieving optimal oncologic results.
While BCS is the treatment of choice for most women with early stage breast cancer (59% of early stage vs. 13% of advanced stage), mastectomies have retained an important role for multi-centric, locally advanced disease (59% of advanced stage vs. 36% of late stage), tumors not visualized on imaging and for deleterious gene carriers (5). Without addressing the axilla, the three main types of mastectomy performed today are total mastectomy (TM), skin sparing mastectomy (SSM) and nipple sparing mastectomy (NSM). TM with or without reconstruction entails removal of the breast with maximal removal of the overlying breast skin. SSM preserves the skin envelope less the nipple areolar complex (NAC) and includes reconstruction. NSM preserves the entire skin envelope and requires some form of reconstruction, typically immediate.
In the United States, we have seen an increasing preference toward mastectomies since 2005, especially among patients with early stage breast cancer (6). Between 2002 and 2012, rates of contralateral prophylactic mastectomies (CPM) more than doubled (3.9% to 12.7%) (7). This trend occurred despite evidence that in most cases, CPM does not increase overall survival (OS), and under National Comprehensive Cancer Network (NCCN) guidelines, CPM is not routinely recommended (8). In spite of improved survival, due in large part to better systemic therapies, improved early diagnosis (9,10) and improved BCS cosmetic outcomes with oncoplastic techniques, we continue to see a trend toward more aggressive breast surgery (11). The preference for mastectomies is likely to be fueled by the availability of improved reconstructive techniques in combination with increased overall patient awareness of surgical options. Appropriate oncologic counseling should be provided for all patients in order to alleviate the fear and anxiety normally associated with their diagnosis. It is our belief that the choice in surgery ultimately lies in the hands of a well-informed patient who is aware of the survival benefit and makes their choice based on understanding not fear or a false sense of safety.
Oncologic safety of nipple and skin sparing mastectomies
Data on the oncologic safety of NSM and SSM has been accumulating for the last 50 years. Early data from Barbara Freeman in 1962 showed a 0.4% incidence of breast cancer at 10-year follow-up in 1,500 subcutaneous mastectomies performed for benign symptomatic fibro-cystic breast disease (12). In 1984, Hinton et al., published their work showing no difference in disease-free survival (DFS) and OS in patients with stage I and II breast cancer undergoing subcutaneous mastectomies (13). There have been multiple large population studies evaluating the safety of prophylactic mastectomies in patients considered to be high risk secondary to family history showing significant decreased incidence of both recurrent and contralateral breast cancers (14-16). In 2013, Agarwal et al. used Surveillance, Epidemiology, and End Results (SEERs) data to compare the DFS and OS following NSM and modified radical mastectomy (MRM) and found no significant difference between the procedures (17). Although their data supported the oncological safety of NSM, they acknowledged possible selection bias (lower tumor grade, negative nodes, no lympho-vascular invasion, etc.), and suggested careful patient selection, as reflected in the NCCN guidelines (8).
Newer data supports preservation of uninvolved nipples regardless of tumor size. Several studies have shown, that while locoregional recurrence is clearly elevated in locally advanced disease, preservation of the uninvolved nipple does not increase the risk of local recurrence and when local regional recurrence does occur, it is not at the nipple (18-20). Smith et al. published their data September 2017, showing that in 2,182 patients with stage 0–III breast cancer who underwent NSM at Massachusetts General Hospital between 2007–2016, at a mean follow-up of 51 months, there was a 2.7% distant recurrence with two deaths and no recurrences at the nipple (19).
Li et al., used SEERS data to look at cancer specific survival (CSS) and OS in 2,440 patients undergoing NSM between 1998–2013 (20). Median age was 50, and they included Tis, T1–3 (79.6% T2–3) and N0–3. Twenty-six percent of the NSM cases were T2–3, 20% were N1–3 and 13.8% were estrogen receptor (ER) negative. For patients diagnosed between 1998–2010 (N=763), median follow-up was 69 months, 5- and 10-year CSS were 96.9% and 94.9% respectively, while OS was 94.1% and 88.0% respectively. Ethnicity, T-stage and N-stage were independently associated with CSS, and age and T-stage were factors independently associated with OS. They showed a 10-year OS of 72% for N2–3 patients after NSM. This was significantly better than the reported outcomes following traditional mastectomy in patients with a similar tumor burden per National Cancer Data-base (8-year OS was 66.6% and 53.5% respectively) following complete mastectomy in N2–3 patients with and without RT, respectively (20).
The above data repeatedly demonstrates that the primary predictor of both locoregional and distant recurrence in breast cancer is the extent of disease at diagnosis, not the surgical approach utilized. Therefore, preservation of the nipple in NSM is a safe option for patients without pathologic evidence of nipple involvement, extensive skin involvement, or the presence of inflammatory cancer.
Prepectoral reconstruction-preoperative selection criteria
Prepectoral reconstruction is not suitable for all patients. Selection criteria for prepectoral reconstruction combine variables that are unique to each individual with conservatively defined general and oncologic safety parameters. In our experience, general disqualifiers for prepectoral reconstruction include patients with ischemic or poorly vascularized mastectomy skin flaps, a history of preoperative breast irradiation (without use of a flap at reconstruction), poorly controlled diabetes (hemoglobin A1c >7.5%), active smoking, immunocompromise, morbid obesity [body mass index (BMI) >40] and lack fat donor sites (Table 1) (21).

Full table
In addition, we have identified a number of oncologic contraindications to the prepectoral reconstructive approach (Table 1) (21). Patients with late-stage breast cancer, posterior tumors involving the pectoralis major muscle, and those at high risk of locoregional recurrence, such as skin nodules or inflammatory disease, should be excluded from prepectoral reconstruction. It is important to note that the oncologic safety of this procedure has not yet been documented over time. As with subpectoral reconstruction, caution should be exercised for large tumors, and for clinically positive lymph nodes (22). We encourage the use of neoadjuvant chemotherapy to downstage the tumor when possible and thus, allow for prepectoral reconstruction in cases of a favorable clinical and radiographic response.
Although a history of radiation therapy negatively impacts prepectoral reconstruction due to compromised tissue perfusion and flap viability, the converse has not been the case. Radiation therapy can and should be used for all prepectoral reconstruction patients with oncologic indication for post-mastectomy radiation. Issues worthy of discussion are the timing of expander or implant placement and possible benefit from using additional flap-based coverage. In our experience, to date, complications associated with adjuvant radiation are not significantly different than those observed with partial tissue or dual plane reconstruction (23). Although complications of skin necrosis can occur, more commonly, complications tend to be skin tightening and stiffness with associated reduction in breast size. Since the modern prepectoral approach is relatively new, oncologic guidelines continue to evolve as long-term data becomes available. We feel that it is prudent to err on the side of caution until data from randomized, prospective clinical trials and results of oncologic outcomes become available.
Prepectoral reconstruction and patient benefit
Prepectoral reconstruction is not a new concept, however, prior versions of this operative technique were limited by aggressive mastectomy dissection, paucity of reconstructive materials and lack of innovative technology. We have made technical advances in the treatment of breast cancer due to the recognition that breast cancer is a disease defined by biology rather than anatomy alone. Bigger surgery does not necessarily translate to better outcome. A new appreciation for existing anatomical divisions between the breast and skin flap hypodermis and a desire for improved mastectomy flap viability, have led to a quest for improving surgical technique. Better understanding of skin flap perfusion and the importance of maintaining appropriate vascular integrity for the success of reconstruction have led to surgical changes that minimize skin flap necrosis. The availability of tissue perfusion assessment devices also allows for objective determination of perfusion and skin flap viability. With their advent, immediate evaluation and decision making regarding a patient’s candidacy for type of reconstruction are now possible. Important to these quantum shifts has also been the use of acellular dermal matrices and materials that stabilize the reconstructed breast and serve as a layer of vascularized regenerative tissue between the implant and mastectomy flap.
A critical question is, why change to prepectoral reconstruction if partial muscle coverage or retropectoral reconstruction are sufficient and have worked well in the past? The answer is derived from patient benefit and satisfaction resulting from an undisturbed pectoralis muscle (24,25). Less trauma to the muscle leads to less pain with decreased need for narcotics and faster recovery. Implant placement over the muscle eliminates animation deformity, improves long term comfort, and lends to a more natural appearing breast (21,24,25). The newly reconstructed breast lies in the natural anatomical position of the surgically removed breast. Further, post-operative complications have been found to be similar for both prepectoral and partial muscle coverage techniques (26-28).
Preoperative planning
Pre-operative surgical planning relies on the input of a multidisciplinary team. In order to maximize outcomes and the patient experience, each specialty must be aware of what the other plans, as our different treatment modalities have potential additive impact on our patients. A team approach leads to better oncologic and aesthetic results as well as improved patient satisfaction. The multidisciplinary team is held by certifying organizations such as the Commission on Cancer (CoC) and National Accreditation Program for Breast Centers (NAPBC) as the sine qua non for the care of the oncologic patient (29). It is imperative that surgeons work in concert with each other in order to achieve the most optimal oncologic outcome along with the best possible aesthetic appearance of the reconstructed breast. While surgeons of the past commonly worked in sequence, today’s surgical oncologist and reconstructive surgeon benefit from working in collaboration.
Communication between the oncologic surgeon and plastic surgeon should start before the mastectomy and continue well beyond the completion of reconstruction. The breast surgeon should convey information to the plastic surgeon regarding oncologic parameters that define reconstructive options. These include the presence of anterior or peripheral tumors potentially compromising the flap or requiring more extensive dissection, ability to preserve the NAC and expectation of adjuvant radiation. The multi-disciplinary discussion should extend to the medical and radiation oncologists who will administer adjuvant or neo-adjuvant treatment. The timing of all therapies should be discussed in advance, as recovery from surgery may interfere with additional treatments planned. Surgical pathology results are best reviewed with a team approach, especially when pathology influences further therapy such as radiation. The timing of second stage reconstruction, if needed, will also be affected by additional treatments such as chemo or radiation therapy. When members of the patient care team are in agreement regarding the treatment plan, patients benefit from focused discussion and the recognition of personalized care.
Patient expectations and involvement in “Enhanced Recovery After Surgery” (ERAS) is also key to optimizing outcomes and patient satisfaction (30,31). Patient education regarding survival benefit, surgical options, unilateral versus bilateral mastectomy and oncoplastic surgery are integral to assuring knowledge and preference driven decision making. Breast surgeons are also foundational in setting proper expectations and covering basic reconstructive techniques at the time of cancer consultation. A well-informed patient has realistic expectations and is able to ask pertinent questions at the time of the reconstructive consultation. Surgeons should be familiar with each other’s postoperative patient instructions in order to avoid conflicting information. Postoperative outpatient visits should be coordinated in a manner that minimizes patient discomfort and inconvenience.
Discussion of post-operative expectations is also important and in line with ERAS recommendations (30,31). We encourage our patients to start pre-operative enteric coated probiotics, 1,000 mg vitamin C, and 81 mg buffered aspirin daily. The aspirin is held 5 days prior to surgery and resumed on post-operative day 1. We discuss expectation of drains and drain care, showering, and negative pressure dressings (32). We encourage use of minimal narcotics combined with nonsteroidal medications for the first few days post-operatively, discussing the expectation of “some discomfort” but minimal “pain”. We also encourage non-particulate liquids up to arrival at hospital rather than the traditional nothing to eat or drink after midnight before surgery. We educate the patient on postoperative constipation exacerbated by narcotics and encourage a bowel regimen to start 3 days preoperatively. With these measures, we have found that many of our patients take few to no postoperative narcotics.
Supportive services such as physical and occupational therapy, as well lymphedema assessment and treatment are included in the post-operative recovery plan. These disciplines are well informed of post-operative limitations in movement so that exercise regimens do not interfere with optimal reconstructive recovery.
Maximizing the mastectomy and placement of incisions
By the time of surgery, decisions regarding the procedure have been made in concert with the patient and derived from vigorous patient education, consideration for patient preference and recommendations of the oncologic team. On the operative day, the breast and plastic surgeon ideally will work in concert. We have found that time spent together in the operating room tends to be mutually educational and enjoyable.
In order to achieve optimal skin flaps while working through limited incisions, the breast surgeon should be aware of the exact location of the tumor and breast boarders. Out lining the breast and anticipated mastectomy field as well as the tumor location with a simple skin marker can be utilized to prevent inadvertent over or under-dissection of flaps and unrelated benign tissue such as the lateral chest wall. These techniques are especially useful for nonpalpable malignancies, or cancers that are located near resection margins. Depending on surgeon comfort and preference, superficial, posterior or peripheral lesions can be identified by intra-operative ultrasound, a marking pin at the overlying skin, or localization of previously placed markers. The ability to confirm complete removal of a peripheral, posterior or axillary tail tumor focus can be critical. If during the procedure, the level of concern for a focal area of involvement extending to a resection margin is high, with potential need for additional resection, send an immediate intraoperative pathology assessment and/or place a small nonabsorbable suture through the full thickness of the flap at the site of concern. With the suture placement technique, if the final pathology is positive for residual disease, you have marked the area for re-excision. If the pathology reveals clear margins, the suture can be easily removed in the office.
Plastic surgeons should plan incision length and location allowing for comfortable dissection by the breast surgeon. Unnecessarily small incisions increase surgical time, tension on flaps, as well as the potential for errors. If blue dye is to be used, both isosulfan blue and methylene blue are acceptable, with the understanding that each can cause skin necrosis. Severe allergic reactions have been observed with isosulfan (33-35). Diluting blue dye and using a limited amount with a slightly subdermal injection will minimize necrosis. From our experience, both dyes can be diluted 1:3 with 0.9 normal saline and work as effectively as full concentration with 0.5 cc single site injected volume. Placement of the injection away from the planned incision lines will further minimize the risk of tissue ischemia. For NSM, place the injection slightly lateral to the NAC and with SSM, place the injection into skin that will be excised.
Incisions for NSM include inframammary, extended inframammary, lateral inframammary, horizontal pericentral (extending from the lateral aspect of the areola), and superior areolar crescent also known as the modified nipple sparing (Figure 1). Our personal preference is the inframammary approach, which has proven to be both effective and safe (36,37). In our opinion, the inframammary incision leads to the most pleasing aesthetic outcome with little or no visible scar with the breast in the upright position. With smaller, ptotic breasts the superior areolar crescent incision allows for removal of superior pole skin resulting in a small lift, while minimizing nipple loss. The best results/nipple viability are seen when the peri-areolar incision is limited to the 9-3 o’clock position. Important issues to consider when choosing incision type are surgeon comfort level with the procedure and the ability to properly remove the breast gland while preserving flap viability. Careful patient selection and use of larger incisions should be encouraged until the operating surgeons masters the procedure and feels comfortable with the technique.
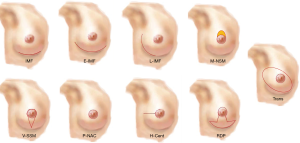
Incisions for SSM include: horizontal central breast, vertical, vertical reduction pattern, and peri-areolar (Figure 1). With the peri-areolar approach, the incision is made along the areolar border, such that the NAC is removed en bloc with the underlying breast gland, and the breast is extracted through this circular incision. If necessary, this incision can be extended superiorly in an elliptical manner, allowing for greater visibility and access, or removal of a larger breast. The peri-areolar approach allows for maximum skin preservation, leaving the reconstructive surgeon the ability to use the entire skin envelope. If needed, reduction of the skin envelope can be achieved by de-epithelialization of the lower pole for overlapping of flaps. For skin-sparing mastectomy, the incisional approach can vary to include the reduction pattern mastectomy, the low horizontal or the vertical incision. The reduction pattern mastectomy allows for a more natural shape of the breast with improved anterior projection and less horizontal expanse. The low horizontal mastectomy allows for the entire incision line to be hidden along the inframammary fold with no flap confluence, decreasing risk for skin necrosis. The vertical incision closely encompassing the NAC and is very useful when the breast is smaller in size, less ptotic or the patient is not a candidate for NSM
If available, perfusion assessment devices allow viewing of post mastectomy perfusion in real-time. These devices have been beneficial in increasing our understanding of the impact of our mastectomy technique and incision placement on flap perfusion. They have also contributed to our appreciation of important perforators, blood flow and areas of increased ischemia susceptibility. Perfusion assessment devices are not only used in real-time decision-making regarding proceeding with prepectoral reconstruction, but also a teaching tool to help the breast surgeon recognize and avoid future dissection errors and eventually be able to identify patients at high risk for failure. For patients with a history of prior breast surgery such as reductions and lumpectomies, these devices can also be used prior to mastectomy to help with optimal incision placement based on perfusion of the pre-mastectomy flap. For instance, a patient with a history of lumpectomy with radiation may have disruption of the medial perforators and have developed a blood flow based on lateral collaterals. By understanding the location of these collaterals, incisions can be placed to avoid disruption of flow and the surgeon can take care to not over dissect thus minimizing the risk of flap necrosis.
Understanding the mastectomy flap and tricks to maximize success
Handling of the mastectomy flap can make the difference between viable and nonviable, success and failure. Retraction damage can be minimized by a gentler touch and the use of non-metal, non-conducting materials. Tumescence, sharp dissection and low thermal conduction devices minimize over-dissection. Leaving extra tissue at the start of the incisions, as well as the area under the nipple will minimize over-thinning during the procedure. Tissue at the edge of incisions tends to be under the most tension and suffers from retraction and friction from the surgeon’s hand. Areas that were purposely left thick at the beginning of the procedure are trimmed once the breast has been removed. Use of proper equipment such as lighted retractors, headlights and appropriately sized instruments ensure a successful operation. The importance of reliable assistance and retraction cannot be overly emphasized.
Overall flap thickness varies by patient weight and size. A very large breasted and obese woman will have a thicker natural flap than a thin, small breasted woman. Adipocytes express hyperplasia and hypertrophy and in obesity both components increase. This leads to an overall enlargement of the thickness of the hypodermis, while the dermal layer remains relatively unchanged (38,39). Frey et al., performed magnetic resonance imagings (MRIs) on 420 NSMs, 379 preoperative and 60 postoperative. The average total preoperative skin/subcutaneous tissue “flap” thickness was 11.4 mm and the average total postoperative flap thickness was 8.7 mm. They found that a flap thickness of less than 8 mm was an independent predictor of ischemic complications. By MRI, the overall postoperative flap thickness was 68.2% of preoperative measurements, and ranged from 52.0% to 74.0% (P<0.0001) (40).
The goal of the oncologic surgeon is to remove the breast gland, not to disrupt the overlying mastectomy flap or its deepest fatty layer, the hypodermis. There is no survival benefit in taking uninvolved tissue beyond the breast gland (39-41). As long as the oncologic surgeon observes the anatomic division between the hypodermis and breast glandular tissue, both thin and thick flaps will remain viable and oncologically sound. The ultimate goal of mastectomy is to remove breast tissue in order to minimize local recurrence. This goal remains the same regardless of the type of reconstruction used, location of tumor, or patient BMI. While, incision placement and flap thickness vary based on the individual, the purpose of the oncologic surgery remains the same.
Summary
Prepectoral implant-based reconstruction offers a safe reconstructive approach holding to all our oncologic principles. It provides superior outcomes in the face of adjuvant radiation therapy, and is patient driven in its acceptance. Prepectoral reconstruction has been proven to provide superior comfort, no motion artifact and a more natural appearing breast with superior symmetry in unilateral mastectomies. While implant rippling remains an issue especially in the thin patient this is seen in dual plain over time as well and can be corrected with fat grafting based on severity. Patients have embraced prepectoral implant–based reconstruction because of its ease of delivery, rapid recovery, and ability to have a reconstruction that is pleasing, comfortable, and well-tolerated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- ACS. Cancer Treatment & Survivorship Facts & Figures 2016-2017. Atlanta: American Cancer Society; 2016.
- ACS. Surgery for Breast Cancer. 2016. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/surgery-for-breast-cancer.html
- De Angelis R, Tavilla A, Verdecchia A, et al. Breast cancer survivors in the United States: Geographic variability and time trends, 2005-2015. Cancer 2009;115:1954-66. [Crossref] [PubMed]
- Steiner CA, Karaca Z, Moore BJ, et al. Surgeries in Hospital-Based Ambulatory Surgery and Hospital Inpatient Settings, 2014: Statistical Brief #223. Available online: www.hcup-us.ahrq.gov/reports/statbriefs/sb223-Ambulatory-Inpatient-Surgeries-2014.pdf
- McCready D, Holloway C, Shelley W, et al. Surgical management of early stage invasive breast cancer: a practice guideline. Can J Surg 2005;48:185-94. [PubMed]
- Kummerow KL, Du L, Penson DF, et al. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 2015;150:9-16. [Crossref] [PubMed]
- Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25:5203-9. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. Breast Cancer Version 4.2017, NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). J Natl Compr Canc Netw 2018;16:310-20. [Crossref] [PubMed]
- Paap E, Holland R, den Heeten GJ, et al. A remarkable reduction of breast cancer deaths in screened versus unscreened women: a case-referent study. Cancer Causes Control 2010;21:1569-73. [Crossref] [PubMed]
- Plevritis SK, Munoz D, Kurian AW, et al. Association of Screening and Treatment with Breast Cancer Mortality by Molecular Subtype in US Women, 2000-2012. JAMA 2018;319:154-64. [Crossref] [PubMed]
- Wong SM, Freedman RA, Sagara Y, et al. Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg 2017;265:581-9. [Crossref] [PubMed]
- Freeman BS. Subcutaneous Mastectomy for Benign Breast Lesions with Immediate or Delayed Prosthetic Placement. Plast Reconstr Surg Transplant Bull 1962;30:676-82. [Crossref] [PubMed]
- Hinton CP, et al. Subcutaneous Mastectomy for Primary Operable Breast Cancer Br J Surg 1984;71:469-72. [Crossref] [PubMed]
- Hartmann LC, Sellers TA, Schaid DJ, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst 2001;93:1633-7. [Crossref] [PubMed]
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of Bilateral Prophylactic Mastectomy in Women with a Family History of Breast Cancer. N Engl J Med 1999;340:77-84. [Crossref] [PubMed]
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral Prophylactic Mastectomy Reduces Breast Cancer Risk in BRCA1 and BRCA2 Mutation Carriers: The PROSE Study Group. J Clin Oncol 2004;22:1055-62. [Crossref] [PubMed]
- Agarwal S, Neumayer L, Agarwal JP. Therapeutic Nipple-Sparing Mastectomy: Trends based on a national cancer database. Am J Surg 2014;208:93-8. [Crossref] [PubMed]
- Burdge EC, Yuen J, Hardee M, et al. Nipple-Sparing Mastectomy is Feasible for Advanced disease. Ann Surg Oncol 2013;20:3294-302. [Crossref] [PubMed]
- Smith BL, Tang R, Rai U, et al. Oncologic Safety of Nipple-Sparing Mastectomy in Women with Breast Cancer. J Am Coll Surg 2017;225:361-5. [Crossref] [PubMed]
- Li M, Chen K, Liu F, et al. Nipple-Sparing Mastectomy in Breast Cancer Patients and Long-Term Survival Outcomes: An analysis of the SEER database. PLoS One 2017;12:e0183448. [Crossref] [PubMed]
- Sigalove S, Maxwell PG, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications and Preliminary Results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Maxwell GP, Storm-Dickerson T, Whitworth P, et al. Advances in nipple-sparing mastectomy: Oncological safety and incision selection. Aesthet Surg J 2011;31:310-9. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove N, et al. Prepectoral Implant-Based Breast Reconstruction and Postmastectomy Radiotherapy: Short-Term Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1631. [Crossref] [PubMed]
- Gabriel A, Sigalove S, Sigalove NM, et al. Abstract P4: Can Surgical Technique Impact Length of Stay and Post-Operative Outcomes in Breast Reconstruction? Plast Reconstr Surg Glob Open 2017;5:104-5. [Crossref]
- Gabriel A, Sigalove S, Sigalove NM, et al. Does Surgical Technique Impact Post-Operative Outcomes of Breast Reconstruction? Available online: https://www.breastsurgeons.org/docs2017/posters/ASBrS_2017_Poster_255754.pdf
- Cordeiro PG, McCarthy CM. A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: Part I. A prospective analysis of early complications. Plast Reconstr Surg 2006;118:825-31. [Crossref] [PubMed]
- Hunsicker LM, Ashikari AY, Berry C, et al. Short-term complications associated with acellular dermal matrix-assisted direct-to-implant breast reconstruction. Ann Plast Surg 2017;78:35-40. [Crossref] [PubMed]
- Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:28-41. [Crossref] [PubMed]
- Fennell ML, Das IP, Clauser S, et al. The Organization of Multidisciplinary Care Teams: Modeling Internal and External Influences on Cancer Care Quality. J Natl Cancer Inst Monogr 2010;2010:72-80. [Crossref] [PubMed]
- Meyer LA, Lasala JL, Iniesta MD, et al. Effect of an Enhanced Recovery After Surgery Program on Opioid Use and Patient-Reported Outcomes. Obstet Gynecol 2018;132:281-90. [Crossref] [PubMed]
- Astanehe A, Temple-Oberle C, Nielsen M, et al. An Enhanced Recovery after Surgery Pathway for Microvascular Breast Reconstruction Is Safe and Effective Plastic and Reconstructive Surgery. Plast Reconstr Surg Glob Open 2018;6:e1634. [Crossref] [PubMed]
- Gabriel A, Sigalove S, Sigalove N, et al. Can Closed Incision Negative Pressure Therapy Impact Post Operative Outcomes in Breast Reconstruction? Plast Reconstr Surg Glob Open 2017;5:46-7. [Crossref]
- Mathes SJ, Nahai F. Reconstructive Surgery: Principles, Anatomy, and Technique. New York: Churchill Livingstone and Quality Medical Publishing Inc.; 1997.
- Schulz S, Zeiderman M, Gunn JS, et al. Safe Plastic Surgery of the Breast II: Saving Nipple Sensation. Eplasty 2017;17:e33. [PubMed]
- Bircan HY, Ozcelik U, Koc B, et al. Cutaneous Necrosis as a Result of Isosulphane Blue Injection in Mammarian Sentinel Lymph Node Mapping: Report of two Cases. Clin Med Insights Case Rep 2014;7:79-81. [Crossref] [PubMed]
- Farahat AM, Hashim T, Soliman HO, et al. Skin sparing mastectomy: technique and suggested methods of reconstruction. J Egypt Natl Canc Inst 2014;26:153-9. [Crossref] [PubMed]
- Rawlani V, Fiuk J, Johnson SA, et al. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. Can J Plast Surg 2011;19:129-33. [Crossref] [PubMed]
- Burns DA, Breathnach SM, Cox N, et al. Rook’s Textbook of Dermatology. 7th edition. Malden, Mass: Blackwell Science, 2004.
- Jo J, Oksana O, Pack S, et al. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol 2009;5:e1000324. [Crossref] [PubMed]
- Frey JD, Salibian AA, Choi M, et al. Mastectomy Flap Thickness and Complications in Nipple-Sparing Mastectomy: Objective Evaluation using Magnetic Resonance Imaging. Plast Reconstr Surg Glob Open 2017;5:e1439. [Crossref] [PubMed]
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 2011;121:2094-101. [Crossref] [PubMed]


