The use of plasmakinetic cautery compared to conventional electrocautery for dissection of abdominal free flap for breast reconstruction: single-centre, randomized controlled study
Introduction
Breast cancer is the most common cancer affecting women in the UK (1). NICE guidelines [2009] recommend reconstruction following mastectomy (2). The abdomen is regarded as an ideal source for reconstructive tissue, because large volumes may be available. In addition, abdominal tissue based reconstruction results in an acceptable donor site scar (3). Therefore, abdominal-based free flaps have become the gold standard method for breast reconstruction following mastectomy (4,5). Although it is a relatively safe procedure (6), extensive dissection of the abdominal tissue may lead to disruption of the normal lymphatic and vascular channels, resulting in seroma development (7). Postoperative donor site seroma can occur in up to 26% of patients undergoing abdominal-based free flap breast reconstruction (8-10). Donor site seroma is problematic in that patients often require multiple outpatient visits for evacuation or aspiration of the seroma. This can prolong recovery, delay wound healing and therefore, potentially interrupt post-operative adjuvant cancer treatment.
Commonly, abdominal flap dissection is performed using a handheld electrocautery device which generates temperatures up to 350 °C for tissue dissection. There are studies that suggest using electrocautery for abdominal tissues dissection can increase incidence of postoperative seroma (11,12). This has prompted surgeons to look for alternative devices such as the plasmakinetic energy device.
The plasmakinetic energy device, PEAK PlasmaBlade (Medtronic, Surrey, UK), dissects tissues at lower temperatures than electrocautery device (40–170 °C) (13). Previous studies suggest that operating at these lower temperatures would result in less inflammation, reduced thermal injury to the adjacent tissues, better wound healing and clinically less wound drainage volume production when compared to electrocautery device (13-15).
This prospective randomized blinded study aims to investigate whether the use of the plasmakinetic cautery confers clinical benefits over conventional electrocautery diathermy in abdominal flap dissection during breast reconstruction.
Methods
This study was approved by the NRES Committee East of England (REC reference: 13/EE/0346). Written informed consent was obtained from all subjects. This study was conducted at St Andrew’s Centre for Plastic Surgery, Broomfield Hospital, Mid Essex Hospital NHS Services Trust, from January 2014 to November 2014.
After ethics approval patients were randomized to either plasmakinetic cautery or conventional electrocautery during the abdominal flap dissection of their breast reconstruction. All patients and assessors were blinded to which cutting instrument was used. All surgery was performed on consecutive patients by the two senior authors (VV Ramakrishnan and M Griffiths). Inclusion and exclusion criteria to the study were specified in Table 1.

Full table
The primary outcome measure was total postoperative drainage volume. Secondary outcome measures were drainage duration, operation time and postoperative complications such as seroma and haematoma. Drainage volume and duration was measured from day 1 post operation to drain removal. Operation time was defined from the skin incision to complete closure of the abdomen. A seroma was defined as a postoperative fluid collection as detected by ultrasonic examination at postoperative day 7 prior to their discharge, and/or at the routine follow-up appointments (2 and 6 weeks). Haematoma was defined as a blood fluid collection, which requires evacuation or aspiration in outpatient patient clinic or return to theatre for bleeding control.
Randomisation
All eligible patients were blindly randomised to either ‘Group 1- Conventional electrocautery’ or ‘Group 2- Plasmakinetic cautery’ with the ratio of 1:1, for the dissection of the abdominal free flap.
Surgical technique
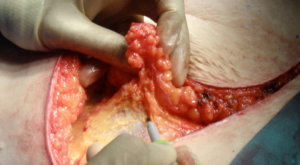
All patients had pre-operative CT-angiogram for surgical planning. All patients received pre-operative prophylactic dose low molecular weight heparin the evening before surgery and continued every evening until the patient was discharged. In all cases, a scalpel was used to make the skin incision down to dermis, then depending on the randomization, conventional cautery or the plasmakinetic cautery (Figure 1) was used to dissect the flap down to scarpa’s fascia. The plasmakinetic device was set to coagulation mode with a range from 6 to 10, and conventional diathermy was used in the coagulation mode with range from 20 to 40. Bleeding vessels were cauterized except for large perforating branches, which were clipped with ligaclips (Ethicon, Johnson & Johnson, UK). Vicryl mesh (Ethicon, Johnson & Johnson, UK) was routinely placed under the rectus sheath on closure. In the abdomen, no quilting sutures were used. Two closed-suction drains were inserted at the inferior aspect of the wound before the closure of the abdominal wound in layers with 3/0 vircyl to the fascia, StratafixTM barbed sutures to dermis and Dermbond topica skin adhesive for wound final approximation. The overall operation time for the dissection of the abdominal flap to complete closure of the abdomen were recorded.
After surgery, all patients were treated according to a standardized protocol. All patients were given intravenous antibiotic for 2 days followed by oral course for 5 days. Abdominal compression garments (9-inch abdominal binder, Marena) were for 6 weeks postsurgery. Abdominal drainage output was recorded daily at 7 am and drains were only removed when drainage was less than 30 mL in 24 hours. The drainage duration over 24 hours was noted. Any complications required return to theatre for intervention such as donor site haematoma and seroma were documented.
Follow-up
Clinical assessment of abdominal wound was performed daily for up to 7 days during their in-patient stay. All subjects had an ultrasound scan of the abdomen at days 7, 14 and 42 post-operation to detect any fluid collection. All non-symptomatic collection was noted with no further intervention performed, unless it became symptomatic. If seroma is detected, this would be aspirated under ultrasound guidance using a needle to dryness, and the volume removed would be recorded.
Statistical analysis
The sample size was calculated based on 5% significance, 80% power, to detect a difference in total abdominal drainage volume by 11%, based on Dogan et al. [2013], who investigated the effect of using plasmakinetic cautery for mastectomy without reconstruction.
Statistical analysis was performed using Prism 7 version 7.0 (GraphPad Software, USA). Continuous variables were compared using t-test and categorical variables were compared using Fisher’s Exact test. Analysis of covariance was used to compare the total drainage volumes between the plasmakinetic cautery and conventional electrocautery groups taking into account the two different surgeons as a separate variable. P<0.05 was used for determination of statistical significance.
Results
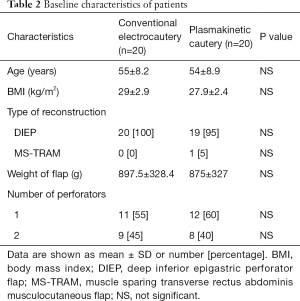
Patient demographics (Table 2)
Full table
Both groups had similar baseline characteristics including age, BMI, type of reconstruction, and weight of abdominal flap harvested. The women were predominantly Caucasian, middle-aged and had a BMI average of 29±2.9 in group 1 (conventional electrocautery) and 27.9±2.4 in group 2 (plasmakinetic cautery). The mean resection flap weights were 897.5±328.4 g in group 1 and 875±327 g in group 2. Mean operation time was 157±50 min in group 1 and 174±70 min in group 2 (P=0.195).
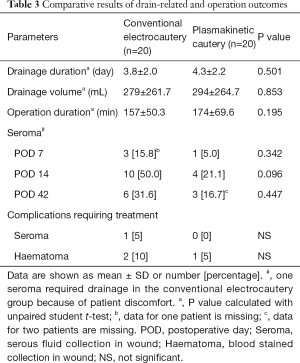
Wound drainage (Table 3)
Full table
Mean drainage volume was 279±262 mL in group 1 and 294±265 mL in group 2 (P=0.853). Similarly, there was no significant difference in the drainage duration between group 1 (4.3±2.2 days) and group 2 (3.8±2.0 days, P=0.501).
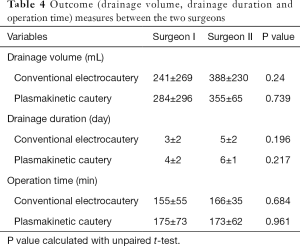
The two different surgeons were factored into the statistical analyses as an additional variable and found there were no significant differences between operation time, drainage volume and drainage duration between the plasmakinetic cautery and conventional electrocautery groups (Table 4).
Full table
Complications
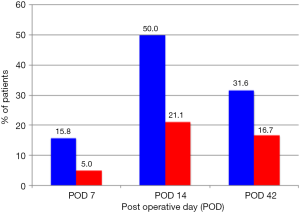
In the conventional electrocautery group, there were 3, 10 and 6 seromas detected at days 7, 14 and 42 post-operation respectively. In the plasmakinetic cautery group, there were 1, 4 and 3 seromas detected at days 7, 14 and 42 post-operation respectively, with no statistical significant differences between the two groups in the prevalence of seroma at any of the time points (Figure 2). One seroma in the conventional electrocautery group required drainage during their outpatient visit at 14 days post-operation. There were 2 haematomas in conventional electrocautery group and 1 haematoma in the plasmakinetic cautery group which required further surgery to evacuate the haematoma.
Discussion
The UK national audit of 1,009 women who underwent mastectomy with immediate or delayed autologous breast reconstruction reported that the most common donor site complications were haematoma and seroma, accounting for up to 8% (16). Nearly 50% of those receiving a mastectomy alone or with immediate reconstruction required drainage of collection, whereas 33% of those undergoing delayed reconstruction required drainage of a collection. In addition, one in four patients in this audit need antibiotics post-discharge for treatment of suspected wound infection, indicating that following mastectomy and breast reconstruction, a significant number of patients suffer post-operative complications.
The purpose of this study was to evaluate if plasmakinetic cautery could improve clinical outcomes in abdominal free flap breast reconstruction surgery. Management of seromas is labour-intensive, which often require multiple outpatient visits for drainage (17). Seromas also increase the risks of developing other wound complications such as infection and wound breakdown (18). Therefore, potential deleterious consequences of seromas include prolonged recovery and delays in receiving any necessary adjuvant chemo/radiotherapy for satisfactory treatment of the breast cancer.
The plasmakinetic cautery used pulsed radiofrequency energy to stimulate a plasma-mediated discharge along the blade, for precise tissue dissection and haemostasis using lower temperature generated than traditional electrocautery, thus reducing collateral thermal damage. This had been demonstrated in studies performed in swine and human abdominal skin (13,14). The present study showed no statistical difference in the drainage volume/duration and operation time in the plasmakinetic cautery group compared to conventional electrocautery group. Not every high-draining wound will lead to development of a clinically apparent seroma, and there may be other less well-understood clinical/biological factors which may determine risk of seroma development. Indeed, the composition of the seroma fluid may warrant future investigation to determine relevant biomarkers associated with improved wound healing (13,14), however, these investigations on the composition of wound drainage fluid are not currently performed normally in clinical practice.
There is only one other clinical study reported in the literature and this was the use of plasmakinetic cautery in breast surgery, which demonstrated in 46 radical mastectomy patients without reconstruction a mean reduction of 386 mL in wound drainage volume, mean reduction of drainage duration by 2.4 days with no increase in operation times and post-operative complications (15). In this published study, the authors believed that the plasmakinetic cautery causes less devitalization of tissue and lysis of subcutaneous fat which had resulted in shorter drainage amount and duration. However, mastectomy and abdominal free tissue flap dissection are very different procedure, the composition of fat contents between breast and abdomen are also very different, which could be the reason for the difference in finding between their paper and the current study.
Seroma following surgery is usually a clinical diagnosis as the majority of small-volume, non-palpable seromas are not routinely identified (19), they can cause further wound problems if they persist (18), which would support the rationale for using ultrasound examination to detect subclinical seromas as in this study. Ultrasonography is an inexpensive, reliable and convenient tool for detection of fluid collections in tissues. It provides a valuable guide for accurate aspiration and drainage of collections, and may be used in outpatient clinics as a useful diagnostic or interventional adjunct to clinical examination (20-23). Di Martino et al. suggested that most seromas were detected at day 14 postoperatively (24,25). Our study showed similar results with 50% of patients in the traditional electrocautery group and 21% of patients in the PEAK PlasmaBlade group developing seromas. While this was not statistical significant it may well be clinically significant and further studies with larger numbers may be required to further investigate this. The seroma incidences in our study were higher than those from Jeevan et al. (16), who published a national survey of complications following mastectomy and breast reconstruction. However in our study, seromas were detected with ultrasound examination which is more sensitive and therefore, higher incidences of seromas would be identified than if only clinical examination was performed. In addition the methods were not clearly described and differences might be attributable to self-reporting of complications.
Patient selection is crucial to reduce postoperative complication in DIEP/MS-TRAM breast reconstruction surgery (26-28). An ideal patient for DIEP breast reconstruction would be a young, non-smoker, BMI <35, non-diabetic and no history of cardiovascular disease (29). Our study was set up with very controlled inclusion and exclusion criteria, such that high-risk patients with co-morbidities that impact on wound healing were excluded. Our study did however show a trend towards seroma detected by ultrasound was increased in the conventional group compared to the plasmakinetic group. It is possible therefore, that the clinical benefit of this device over conventional diathermy would be more apparent if the study was conducted in a larger cohort of patients, broader inclusion criteria more relevant to the general population of patients that we would treat, as would including multiple centres that perform abdominal based free flap reconstruction.
If the use of this new dissection device in DIEP/MS-TRAM breast reconstruction surgery would reduce complications such as seroma and haematoma, this could lead to a cost saving from needing further hospital visits and hospitalization. In addition this might have applications beyond breast reconstructive surgery, in other aesthetic realm where extensive tissue dissection is required. This device could be used for procedures such as abdominoplasty or body contouring surgeries following massive weight lost.
Conclusions
This study demonstrated although there was no significant difference in the donor site drainage volume/duration, and overall postoperative incidence of seroma/haematoma between the two randomized groups, there was a trend towards less seromas in the plasmakinetic cautery group. This is in keeping with other studies in the literature. Future randomized controlled studies with larger sample size would be required to show whether the plasmakinetic cautery confers a postoperative benefit for abdominal flap dissection in breast reconstruction surgery over conventional electrocautery.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the NRES Committee East of England (REC reference: 13/EE/0346). Written informed consent was obtained from all subjects.
References
- Cancer Research UK. Breast cancer incidence statistics. 2014; Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/incidence-invasive. Accessed April 5, 2016.
- National Institute for Health and Care Excellent. NICE guidance2009. Early and locally advanced breast cancer: diagnosis and treatment. Available online: https://www.nice.org.uk/guidance/CG80/chapter/1-Guidance#breast-reconstruction. Accessed April 5, 2016.
- Niddam J, Bosc R, Lange F, et al. DIEP flap for breast reconstruction: retrospective evaluation of patient satisfaction on abdominal results. J Plast Reconstr Aesthet Surg 2014;67:789-96. [Crossref] [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg 1999;52:104-11. [Crossref] [PubMed]
- Zoghbi Y, Gerth DJ, Tashiro J, et al. Deep Inferior Epigastric Perforator Versus Free Transverse Rectus Abdominis Myocutaneous Flap: Complications and Resource Utilization. Ann Plast Surg 2017;78:516-20. [Crossref] [PubMed]
- Fang RC, Lin SJ, Mustoe TA. Abdominoplasty flap elevation in a more superficial plane: decreasing the need for drains. Plast Reconstr Surg 2010;125:677-82. [Crossref] [PubMed]
- Salgarello M, Tambasco D, Farallo E. DIEP flap donor site versus elective abdominoplasty short-term complication rates: a meta-analysis. Aesthetic Plast Surg 2012;36:363-9. [Crossref] [PubMed]
- Venkat R, Lee JC, Rad AN, et al. Bilateral autologous breast reconstruction with deep inferior epigastric artery perforator flaps: Review of a single surgeon's early experience. Microsurgery 2012;32:275-80. [Crossref] [PubMed]
- Seidenstuecker K, Munder B, Mahajan AL, et al. Morbidity of microsurgical breast reconstruction in patients with comorbid conditions. Plast Reconstr Surg 2011;127:1086-92. [Crossref] [PubMed]
- Swanson E. Reducing seroma rates after abdominoplasty by avoiding electrodissection. J Plast Reconstr Aesthet Surg 2015;68:864-5. [Crossref] [PubMed]
- Rousseau P, Vincent H, Potier B, et al. Diathermocoagulation in cutting mode and large flap dissection. Plast Reconstr Surg 2011;127:2093-8. [Crossref] [PubMed]
- Loh SA, Carlson GA, Chang EI, et al. Comparative healing of surgical incisions created by the PEAK PlasmaBlade, conventional electrosurgery, and a scalpel. Plast Reconstr Surg 2009;124:1849-59. [Crossref] [PubMed]
- Ruidiaz ME, Messmer D, Atmodjo DY, et al. Comparative healing of human cutaneous surgical incisions created by the PEAK PlasmaBlade, conventional electrosurgery, and a standard scalpel. Plast Reconstr Surg 2011;128:104-11. [Crossref] [PubMed]
- Dogan L, Gulcelik MA, Yuksel M, et al. The effect of plasmakinetic cautery on wound healing and complications in mastectomy. J Breast Cancer 2013;16:198-201. [Crossref] [PubMed]
- Jeevan R, Cromwell DA, Browne JP, et al. Findings of a national comparative audit of mastectomy and breast reconstruction surgery in England. J Plast Reconstr Aesthet Surg 2014;67:1333-44. [Crossref] [PubMed]
- Kuroi K, Shimozuma K, Taguchi T, et al. Pathophysiology of seroma in breast cancer. Breast Cancer 2005;12:288-293. [Crossref] [PubMed]
- Sadeghi A, Malata C. CASE REPORT Persistent Seromas in Abdominal Free Flap Donor Sites After Postmastectomy Breast Reconstruction Surgery: Case Reports and Literature Review. Eplasty 2013;13:e24. [PubMed]
- Mohammad JA, Warnke PH, Stavraky W. Ultrasound in the diagnosis and management of fluid collection complications following abdominoplasty. Ann Plast Surg 1998;41:498-502. [Crossref] [PubMed]
- Ahmed M, Abdullah N, Cawthorn S, et al. Why should breast surgeons use ultrasound? Breast Cancer Res Treat 2014;145:1-4. [Crossref] [PubMed]
- Thomason K, Cooke PH. The use of surgeon-performed ultrasound assessment in a foot and ankle clinic. Foot Ankle Surg 2012;18:213-5. [Crossref] [PubMed]
- Bennett IC, Biggar MA. The role of ultrasound in the management of breast disease. Australas J Ultrasound Med 2011;14:25-8. [Crossref] [PubMed]
- Lee SH, Kim YJ. Managing a seroma with wireless mobile ultrasound device. J Plast Reconstr Aesthet Surg 2017;70:e7-9. [Crossref] [PubMed]
- Di Martino M, Nahas FX, Barbosa MV, et al. Seroma in lipoabdominoplasty and abdominoplasty: a comparative study using ultrasound. Plast Reconstr Surg 2010;126:1742-51. [Crossref] [PubMed]
- Di Martino M, Nahas FX, Kimura AK, et al. Natural evolution of seroma in abdominoplasty. Plast Reconstr Surg 2015;135:691e-8e. [Crossref] [PubMed]
- Chang DW, Wang B, Robb GL, et al. Effect of obesity on flap and donor-site complications in free transverse rectus abdominis myocutaneous flap breast reconstruction. Plast Reconstr Surg 2000;105:1640-8. [Crossref] [PubMed]
- Chang DW, Reece GP, Wang B, et al. Effect of smoking on complications in patients undergoing free TRAM flap breast reconstruction. Plast Reconstr Surg 2000;105:2374-80. [Crossref] [PubMed]
- Scheer AS, Novak CB, Neligan PC, et al. Complications associated with breast reconstruction using a perforator flap compared with a free TRAM flap. Ann Plast Surg 2006;56:355-8. [Crossref] [PubMed]
- Garcia-Tutor E, Murillo J. The ideal patient for the first breast reconstruction using a diep flap. Plast Reconstr Surg 2003;111:947-8. [Crossref] [PubMed]