Evolution of radiotherapy techniques in breast conservation treatment
Introduction
A year after Wilhelm Röntgen discovered X-rays in 1895, radiation was used by Emil Grubbe in Chicago to treat a patient with inoperable breast cancer, using radiation energies that could penetrate at most a superficial skin cancer today (1). In January 1896, Grubbe (manufacturer of Crookes Cathode Ray Tubes) treated Mrs Rose Lee, a patient with locally advanced breast cancer, after the idea was prompted by a physician who reviewed Grubbe’s hand which had radiation dermatitis, epilation and ulceration from his experiments.
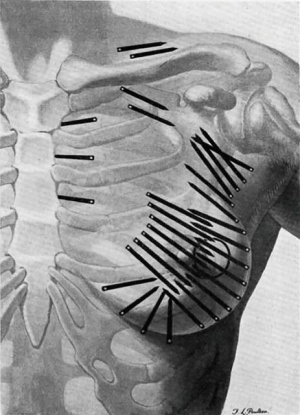
In the 1920s, radium was used following conservative surgery by surgeon Geoffrey Keynes at St. Bartholomew’s, London, producing equivalent survival rates to Halsted’s radical mastectomy, introduced in 1896. Keynes compared his technique to Halsted’s, writing that “unnecessarily drastic operations are thereby eliminated, and to many women the saving of the breast is of the greatest psychological significance” (Figure 1) (2,3).
Following World War 1, “deep X-ray” was introduced, with energies up to 200 kV. Megavoltage linear accelerators and cobalt beam were largely introduced in the 1960s (4). However, radiation therapy (RT) remained imprecise, with lower energies causing severe skin reactions, and planning involving surface anatomy, gentian violet marks and tracing paper, rather than the sophisticated imaging and 3D-computing techniques of today.
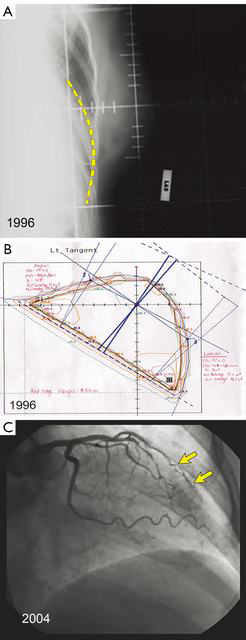
Techniques have evolved since the 1980s when radiotherapy plans were often done at the center of the treated breast known as the “central axis” [two dimensional (2D) plans] with plain X-ray verification films. Later plans were done with an additional slice at the superior and inferior ends of the field (Figure 2A,B). With the advent of supercomputing and improved software algorithms, 3D plans allow better dose uniformity across the breast that reduces areas of fibrosis and potentially breast edema, as well as reduced heart and lung doses (Figure 2C). Further, there is better understanding about the various pathologic sub-types of invasive cancer and the risk of local recurrence, with ongoing trials evaluating hormone therapy alone after conservative surgery for selected, biologically low-risk tumors (5-9).
Initial trials of breast conservation using standard fractionation
Moving from traditional mastectomy to breast conservation took time, as many clinicians could not believe that conservation was equivalent to more extensive surgery. Mastectomy rates varied significantly by institution and surgeon. Dedicated breast surgeons had lower mastectomy rates than general surgeons, and mastectomy rates were usually lower in urban areas (10). Making this change required the efforts of individual clinician champions, breast cancer advocacy groups, Government investment in guideline development and the media.
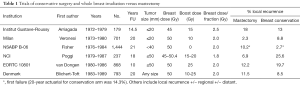
The initial trials of breast conservation compared the procedure of total mastectomy and axillary dissection to various breast conservation techniques with radiation (11-16). Table 1 shows the vast differences in local recurrence rates which, in retrospect, were largely due to poor selection criteria, poor imaging and often positive margins. However, these trials became the foundation of future trials that modified their surgical and radiation techniques and numerous non-randomized trials that showed excellent local control rates without a mastectomy (17). Further, they clearly showed that mastectomy did not improve survival rates; indeed, in some sub-groups, such as node-positive patients, mastectomy was inferior to breast conservation (15).
Full table
Selection criteria for RT
There are no specific contraindications to breast conservation based on age or family history or stage of disease, but radiation must be avoided or delayed for patients who are pregnant, and care should be taken for patients with active collagen vascular diseases. Finally, some patients want to avoid radiation if possible and prefer a mastectomy after informed consent. A mastectomy or initial neoadjuvant chemotherapy is indicated for larger tumours that cannot be removed with an acceptable cosmetic result. In the 1980s and 1990s, many factors that can increase the risk of a recurrence in the breast after conservative surgery and radiation were identified, such as very young age at diagnosis, an extensive intraductal component (EIC), lymphatic vessel invasion and, importantly, margins of resection (17-19). Recent guidelines defining a clear margin as no ink on the surgical edge will hopefully reduce the risk of unnecessary re-excisions, particularly given falling local recurrence rates with better imaging and patient selection, and the use of chemotherapy, hormonal treatment (HT) and trastuzumab (Herceptin®) (20-22). A Danish study of 11,900 patients found no evidence of improved local control by margin depth (23).
When whole breast radiation is required, it should be given with the minimum delay possible (usually 3–4 weeks), particularly for patients not receiving chemotherapy. Assuming a 10-year risk of breast cancer recurrence of 6%, it is estimated that the absolute risk is increased by a further 0.5% for every month treatment is delayed (24).
The role of a radiation boost to the primary tumor site
Detailed pathologic studies done by Holland in the 1980’s found that, irrespective of primary tumor size, about 40% of patients had cancer cells 2 cm beyond the edge of the primary tumor and 11% of patients had cancer cells as far as 4 cm away from the edge of the primary tumor. The residual tumor was often ductal carcinoma in situ (DCIS) as well as cancer in the lymphatics (25). Further, early studies of the recurrence patterns after conservative surgery and RT found that 60–70% occurred at the primary site, identifying this as an important focus area for clinical examination, additional mammographic views, ultrasound and non-surgical biopsy after treatment (18,26).
In a long-term retrospective study from Westmead Hospital, most patients who developed recurrent breast cancer did so in the region of the original primary site. Most recurrences in the breast were within the scar or boost region with a median time to recurrence of 38 months (range, 8–127 months). Recurrences that occurred elsewhere in the breast accounted for 31% of all recurrences and tended to occur later, with a median follow-up of 56 months (range, 30–101 months). Long-term follow-up is therefore important when evaluating partial breast irradiation techniques (26).
A European EORTC trial of over 5,000 patients reported a 20-year breast tumor recurrence rate of 16.4% in the no-boost group versus 12.0% in the boost group (P<0.0001) (27). The boost significantly reduced the risk of a recurrence for young patients or those with an EIC or a grade 3 tumor. For patients with grade 3, ER-negative tumors, the boost reduced the risk of a breast tumor recurrence from 31% to 5% (P=0.01), highlighting the need to boost biologically more aggressive tumors irrespective of fractionation schedule (28). Given the fall in local recurrence, not all patients require a boost (29,30). A Cochrane review of the utility of a boost can be found elsewhere (31).
A previous study by Boyages found that patients treated without a boost (usually because of a large excision specimen or a negative re-excision) had no increase in the risk of local recurrence compared to patients who did receive a boost, and doses to the primary tumor site above 60 Gy did not improve local tumor control (26). In contrast, re-excision was associated with a higher risk of local recurrence in the Danish margin study and the authors postulated that the increased delay in commencing adjuvant therapy or inadequate re-excision due to poor orientation of the initial specimen could be contributing factors (23). Adams found a similar result, where six of the 64 re-excised patients (9.4%) had a recurrence in the breast versus 12 of the 459 non-re-excised patients (2.6%) (P=0.02) and argued that this is most likely due to tumor biology (32). Despite this finding, a large retrospective study from the Netherlands found that patients who had a re-excision and conservation, or re-excision followed by a subsequent mastectomy had identical survival rates to patients who underwent breast conservation in one procedure (33).
There is a danger with modern voluming that potential under-dosing can occur of the primary tumor bed compared to older techniques. Important issues to consider when planning a boost PTV include the following:
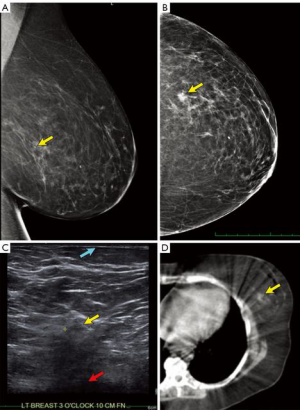
- Understand the exact location of the tumor by examining the pre-operative mammogram (Figure 3A,B), ultrasound (Figure 3C) and breast MRI, if available. Particularly understand how close to the chest-wall or skin the tumor is located, as tight radiation margins may lead to under-dosing.
- Correlate the depth of the tumor using pre-operative breast ultrasound of the index lesion. Usually the relationship of the tumor to the skin can be observed (Figure 3C).
- Examine the pre-operative lymphoscintigram images which often include a CT scan (Figure 3D). The primary breast tumor is sometimes clearly visible and distances from the midline and anterior/posterior position more clearly visible.
- Examine any pre-operative CT or PET-CT scans of the thorax to locate the breast lesion (34). An example of a tumor close to the pectoral fascia is shown in Figure 4.
- Patients with tumors abutting the chest-wall should include a 3–5 mm margin into the lung to avoid under-dosing of the posterior aspect of the tumor, which is often removed with very close pathologic margins.
- Carefully read the macroscopic section of the pathology report to determine if extra shaves of tissue were taken around the initial excision and correlate this with the final excision margins. It makes sense to use wider expansions in areas of closer margins.
- Determine if there are risk factors for local recurrence such as extensive lymphatic invasion, an EIC and possibly very young patient age, where a more generous boost should be used.
- Review planning CT scan and note any seroma which may delineate tumor cavity or preferably the location of titanium clips placed at the surgical cavity (Figure 5). Our practice is to routinely place titanium clips onto the walls of the excision cavity (medial, lateral, superior and inferior aspects) at the 3, 6, 9 and 12 o’clock position and at the center of the excision cavity on the pectoralis fascia prior to any breast remodeling. More details are found elsewhere (35).
- For patients where there is risk of sub-clinical nipple duct involvement, such as those with high-grade DCIS close to the nipple, attention to the margin extending towards the nipple should be considered (36).
- It is insufficient simply to “boost the scar”, because with modern oncoplastic techniques, the scar often has no relationship to the actual primary tumor site where the risk of recurrence is highest (35). Different boost techniques include electron beam, which creates more skin reaction, or an external photon boost, which may involve treating more normal tissue, or more localized synchronous boost techniques.

Marking the boost region on a planning CT scan of the breast appears simple, but poor technique can potentially increase the risk of local relapse. In one study, which assessed the RTOG breast voluming atlas, there was marked variation in the boost planning target volume (Boost_PTV) but less variation in the boost gross tumor volume (Boost_GTV), which essentially circled the lumpectomy cavity seroma and any associated breast surgical clips (37). One drawback of the RTOG atlas is the lack of clarity regarding definition of the PTV. In a similar study from the Netherlands with documented guidelines which included a total margin from the CTV of approximately 20 mm, inter-observer variation was shown to be substantial (SD 2–12 mm) for the clinical target volume (CTV), despite delineation guidelines (38).
With all the above caveats, the NRG Oncology NSABP protocol B-51/RTOG protocol 1304, boost guidelines are useful.
Lumpectomy GTV: represented the surgical cavity from the breast conserving surgery with contouring using all available clinical and radiographic information including the excision cavity volume, architectural distortion, lumpectomy scar, seroma and/or extent of surgical clips.
Lumpectomy CTV: consists of the contoured lumpectomy plus a 10-mm 3D expansion with the following 3 limitations: (I) CTV limited posteriorly at anterior surface of the pectoralis major; (II) antero-laterally 5 mm from skin; and (III) CTV should not cross midline (39).
Lumpectomy PTV: this is a 7-mm expansion on the Lumpectomy CTV and excludes the heart (Figure 5). Therefore the potential total expansion from the GTV was 17 mm, similar to the 20 mm above for the Netherland study (40,41). We use a 10 rather than 7 mm expansion for patients at higher risk of a local recurrence, e.g., with extensive lymphovascular invasion present.
Conservative surgery alone
One way of reducing radiation toxicity has been to reduce the volume of the breast receiving radiation. The most extreme form of this is to recommend no radiation at all. In Fisher’s NSABP-B06 study, the incidence of an ipsilateral breast recurrence was 14.3% for women who underwent lumpectomy and breast irradiation, compared to 39.2% for women who underwent lumpectomy alone (P<0.001). RT was associated with a marginally significant decrease in deaths due to breast cancer [hazard ratio (HR) 0.82; P=0.04]. At 20 years, there were 44 more deaths from breast cancer for patients who had lumpectomy alone than for those who had lumpectomy and radiation, equating to a 10% difference in breast cancer death rate (11). By collating individual data from multiple trials, the Oxford overview confirmed what we expected: with time, higher recurrence rates were associated with subsequent death rates. After conservative surgery, RT to the breast halves the overall recurrence rate and reduces breast cancer mortality by about one-sixth (Table 2) (42).

Full table
Several trials attempted conservative surgery firstly with or without RT, and later, with or without HT, usually tamoxifen (TAM). Although recent trials have reported lower recurrence rates, this is partly due to shorter follow-up and better patient selection and may not generally apply to a community setting. The Oxford overview found that older patients with small, low-grade, oestrogen-receptor (ER) positive tumors have a low recurrence rate and are possible candidates to omit RT in some clinical circumstances (42) and others have tested accelerated partial breast irradiation (APBI) in this low-risk group (43). Boyages and others have recently reviewed trials of lower-risk older patients with small, ER-positive, node-negative tumors randomized between conservative surgery +/− RT and/or HT (44,45). Adjuvant HT reduces the risk of an in-breast recurrence when compared with surgery alone, but the combination of RT +/− HT is more effective and more sustainable (11,46-51). However, for highly selected patients with wider margins, minimal associated DCIS and no lymphatic invasion or other risk factors, this option should be discussed. If implemented, it is critically important to ensure close clinical and imaging follow-up for at least 10–15 years. The 15-year data of the Swedish sector resection versus sector resection + RT found a local recurrence of 23.9% versus 11.5% (P<0.001) with associated lower overall survival of 68.4% versus 71.1% (P=0.68) (52). For patients who want more “insurance”, a short hypofractionated technique over 3 weeks, or APBI technique over 2 weeks is probably better than no radiation.
Accelerated whole breast irradiation (AWBI)
Hypofractionation involves a shorter or accelerated radiation course with higher daily doses for the same biological effect (Table 3) (53-56). AWBI saves the patient time and money and reduces pressure on public radiotherapy units. The UK Standardization of Breast Radiotherapy (START)-A trial compared standard 50 Gray (Gy) in 25 attendances (fractions) to 41.6 or 39 Gy in 13 fractions. In START-B, 50 Gy in 25 fractions over 5 weeks was compared with 40 Gy in 15 fractions over 3 weeks (54) similar to another UK study from the West Midlands Oncology Group (55). A Canadian trial for node- and margin-negative disease compared 42.5 Gy in 16 fractions to 50 Gy in 25 fractions (56). Boost was optional in the UK trials and omitted in the Canadian trials. Shorter schedules were not worse for local recurrence or survival compared to the control group (4.7%, P=0.01), except for high-grade tumors (15.6% 10-year local recurrence), perhaps due to the lack of a radiation boost in the Canadian trial. More detailed analysis and a longer follow-up of the Canadian study found that grade alone did not influence local recurrence, which was lower for low-grade ER-positive tumors (4.5%) and higher-grade triple-negative breast cancer (4.5%), but statistically higher (P<0.001) for higher-grade ER-positive tumors (7.9%) and those with HER2-positive tumors (6.9%) (57). The START trials did not demonstrate a higher local recurrence rate for higher-grade tumors, perhaps because 61% received an additional RT boost.
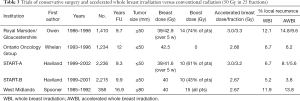
Full table
In practice, standard fractionation appears to be better for larger-breasted women for whom increased breast oedema can be a problem. It remains to be seen if this can be reduced with more sophisticated radiation techniques. In the Canadian trial, women with large breasts were excluded, few women received chemotherapy and nodal radiation was not given. In the START trial, cosmetic result was not inferior for larger-breasted women, but only a small number were in this group (15.6%). However, a recent study from the MD Anderson Cancer Center randomized 287 women, 40 years or older with stage 0 to II breast cancer for standard fractionation to the whole breast [50.00 Gy/25 fractions + boost (10.00–14.00 Gy/5–7 fractions)] vs. AWBI [42.56 Gy/16 fractions + boost (10.00–12.50 Gy/4–5 fractions)]. Of note, 76% of study participants were overweight or obese and large breast size was not a contraindication to treatment. Treatment with AWBI yielded lower rates of acute effect than WBI, as well as less fatigue and less difficulty meeting family needs 6 months after completing RT. Longer follow-up is required to assess late outcomes such as fibrosis and cosmesis, although it is highly probable that with modern planning techniques, such as intensity modulated radiation therapy (IMRT) or volumetric modulated arc therapy (VMAT), reduced-dose inhomogeneity will lead to reduced toxicity for larger-breasted women (58). Studies of five treatments (one per week for five weeks) are also being evaluated. With a median follow-up of 37.3 months, two local tumor relapses occurred and 28.5 Gy in 5 fractions was found to be comparable to 50 Gy in 25 fractions and significantly milder than 30 Gy in 5 fractions in terms of adverse effects in the breast (59).
Recent meta-analyses of published and unpublished studies of hypofractionation include 13 trials with 8,189 participants. No differences were observed in local failure (7 trials), loco-regional failure (8 trials) or survival (4 trials). Further, hypofractionated RT was associated with significantly less acute toxicity, but no difference in late cosmesis (60,61).
The Australian and US guidelines only recommend hypofractionated treatment for patients 50 years or older who have node-negative tumors up to 5cm, have had no chemotherapy and where breast size and treatment technique minimises dose variation across the volume (62,63) and the Australian guidelines advise caution when recommending this approach to other patients. The UK NICE guidelines recommended that all patients receive 40 Gy in 15 fractions (64).
A pragmatic approach is to prescribe 42.5 Gy in 16 fractions if a boost is not required (e.g., negative re-excision, unicentric luminal A tumor in a patient over 70 with negative margins and no lymphatic vessel invasion). If a boost is required, 40 Gy in 15 fractions followed by 10 Gy in 4 or 5 fractions is acceptable. A German group has tested 40 Gy in 16 fractions for a synchronous integrated boost of an additional 0.5 Gy per fraction to the tumor bed for a total dose of 48.0 Gy in 16 fractions with good tolerance and minimal complications (65).
APBI
The utility of APBI is not yet entirely clear, largely because of the still relatively short follow-up in these studies. However, it is an option for older patients with small, unicentric good prognosis tumors with negative margins. This type of radiation can be delivered using several techniques: (I) interstitial brachytherapy (high dose rate, pulsed dose rate, permanent implants); (II) brachytherapy using the balloons (Mammosite®, Contura®); (III) hybrid brachytherapy devices [strut-adjusted volume implant (SAVI) applicator]; (IV) external beam radiotherapy (3D, IMRT, VMAT); or (V) intraoperative radiotherapy with electrons or X-rays, (detailed below). A review of these techniques and associated controversies has been reported elsewhere (66,67).
The Groupe Europeen de Curiethrapie-European Society of Therapeutic Radiology and Oncology (GEC-ESTRO) randomized 1,184 patients to whole breast irradiation or interstitial APBI. Between 2004 and 2009, 551 patients had whole-breast irradiation with tumor-bed boost and 633 patients received APBI using interstitial multicatheter brachytherapy (68).
Patients were considered eligible for the trial if they were aged 40 years or older, had localized pTis or pT1–2a (lesions of ≤3 cm diameter), pN0/pNmi, and M0 breast cancer, had undergone local excision of the breast tumor with microscopically clear resection margins of at least 2 mm in any direction (in cases of invasive lobular carcinoma or DCIS, at least 5 mm), and had no lymphovascular invasion.
APBI was delivered with high-dose-rate (HDR) or pulsed-dose-rate (PDR) multicatheter brachytherapy. For HDR brachytherapy, a total dose of 32.0 Gy in eight fractions or 30.3 Gy in seven fractions, with fractionation twice a day was prescribed. For PDR brachytherapy, a total of 50 Gy with pulses of 0.60–0.80 Gy/hour (one pulse per hour, 24 h/day) was given. Patients allocated irradiation of the whole breast were delivered a total dose of 50.0–50.4 Gy in 25–28 fractions. An electron boost of 10 Gy in five fractions was also given.
The 5-year local recurrence rate was 1.4% for APBI and 0.9% for whole-breast irradiation (P=0.42). Five-year toxicity profiles and cosmetic results were similar in patients treated with breast-conserving surgery followed by either APBI with interstitial brachytherapy or conventional whole-breast irradiation, with significantly fewer grade 2–3 late skin side-effects after APBI with interstitial brachytherapy (69).
GEC-ESTRO also conducted a similar trial using IMRT techniques for the APBI arm. In this study, for patients assigned to the APBI arm, the CTV was drawn with a uniform 10 mm three-dimensional margin around the surgical clips and was limited to 3 mm from the skin surface. A second uniform three-dimensional 10 mm margin was added to the CTV to obtain the PTV. The PTV could extend 4 mm inside the ipsilateral lung. A dose of 30 Gy in five non-consecutive daily fractions at 6 Gy/fraction was prescribed over 2 weeks.
The following constraints were adopted: 100% of PTV covered by 95% of the prescribed dose (V28.5 =100%); maximal dose to PTV <105% (31.5 Gy); minimal dose to PTV 28 Gy (93.3%); un-involved ipsilateral breast: (V15 <50%); ipsilateral lung (V10 <20%); contralateral lung (V5 <10%); contralateral breast, maximal dose <1 Gy; and heart (V3 <10%). The 5-year IBTR rate was 1.5% (three cases) in the APBI group and 1.4% (three cases) in the whole breast irradiation group (P=NS). The APBI group presented significantly better results considering acute (P=0.0001), late (P=0.004), and cosmetic outcome (P=0.045) (70). A trial from Barcelona also used external beam irradiation for the APBI arm, which significantly reduced the complexity of this technique (71).
In the UK-Import-Low trial, patients assigned to whole-breast radiotherapy (control) received 40 Gy in 15 fractions, those assigned to the reduced-dose group received 36 Gy in 15 fractions to the whole breast and 40 Gy in 15 fractions to the partial breast containing the tumor bed, and those assigned to the partial-breast group received 40 Gy in 15 fractions. In this study, the GTV (seroma or area of titanium clips) was expanded by 15 mm to obtain the CTV and a further 10 mm for the PTV. Anteriorly, the PTV was 5 mm from the skin surface and did not extend beyond the pectoral fascia posteriorly and/or was no more than 5 mm from the lung/chest wall interface. The partial-breast radiotherapy technique used standard tangential fields that were simply shortened to encompass the PTV, which meant that while a larger volume of breast is treated than with other 3D conformal or IMRT and brachytherapy techniques, tangential beams minimise the dose to surrounding organs at risk, such as the heart and lungs, by keeping the exit beams within the breast. Breast hardness was reported by patients in 35%, 21% and 15% of the whole-breast control, whole breast (reduced dose group) and the partial breast groups respectively (72).
Local recurrence and late normal-tissue effects were uncommon in all groups (Table 4). Significantly fewer patients reported breast hardness in the partial-breast radiotherapy group compared with the control. An unexpected problem of PBI is that there is a higher incidence of recurrence in the axilla, as whole breast irradiation also partially treats level 1 and 2 of the axilla (73).
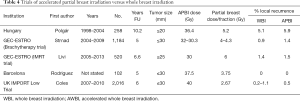
Full table
The American Society for Radiation Oncology (ASTRO) has updated its consensus statement on the use of APBI with the “Suitable” group now including the following patient and tumor factors: age ≥50 y; margins negative by at least 2 mm; T stage: Tis or T1 or screen-detected low to intermediate nuclear grade DCIS ≤2.5 cm resected with margins negative at ≥3 mm (74).
Intraoperative RT
As noted above, intraoperative radiation is a type of partial-breast irradiation. To date, two randomized clinical trials (the ELIOT and TARGIT trials) have investigated the use of intraoperative radiotherapy for delivery of APBI (75,76). One of the difficult issues in this type of approach is that the final pathology report is not available as the therapy is given. The ELIOT study was done at the European Institute of Oncology (Milan, Italy) where 1,305 female patients aged 48 to 75 years who had a maximum tumor diameter of up to 2.5 cm and were suitable for breast-conserving surgery were randomly assigned to receive either whole-breast external radiotherapy or intraoperative radiotherapy with electrons. Patients in the intraoperative radiotherapy group received one dose of electrons to 21 Gy to the tumor bed at the time of surgery. Those in the external radiotherapy group received 50 Gy in 25 fractions of 2 Gy, followed by a boost of 10 Gy in five fractions. After a median follow-up of 5.8 years, the 5-year event rate for an ipsilateral breast tumor recurrence was 4.4% for the intraoperative radiotherapy group and 0.4% for the external radiotherapy group (HR 9.3). Five-year overall survival was not significantly different between the two groups and approached 97%. There were significantly fewer skin side-effects in women in the intraoperative radiotherapy group than in the external radiotherapy group (P=0.0002).
The TARGIT trial recruited patients between 2000 and 2008 and randomized them between a control arm of whole breast radiation of 40 to 56 Gy in 15–25 fractions (+/− Boost of 10–16 Gy in 5–8 fractions) and an intraoperative technique using low-energy X-rays of 50 kV. The prescribed dose was 20 Gy in one fraction to the applicator surface, which corresponded to 5–7 Gy at 1 cm from the applicator. After a median follow-up of 24.6 months, the Kaplan-Meier estimate of local recurrence at 4 years did not differ between TARGIT (1.20%) and control (0.95%) (77). However, in an updated report, the 5-year risk of local recurrence was significantly greater in the TARGIT group (3.3% vs. 1.3%; P=0.042) (75). Several concerns about this trial have been discussed elsewhere but the technique has been accepted as a UK NICE guideline with some controversy (78-81).
The internal mammary chain-balancing treatment with cardiac irradiation
IMC RT can reduce distant metastases and potentially improve survival, as shown in the Oxford overview and recent trials (Table 2) (42,82-88). For example, the MA.20 trial randomized patients with node-positive or high-risk, node-negative disease treated with conservative surgery and RT to additional RT to the regional nodes, including the IMC (88). Distant metastases were 12.9% in the nodal-irradiation group and 16.5% in the control group (P=0.03) with no difference in breast cancer mortality. Patients with ER-negative disease who had IMC RT had a lower 10-year mortality rate than patients without IMC RT (18.7% vs. 26.1%) (P=0.05). Other trials show similar findings and support the notion that leaving IMC disease behind is probably detrimental to survival and that this area should be selectively treated using RT, as addressed elsewhere (44).
IMC radiation is difficult because it potentially increases heart dose, morbidity and mortality. There is also variation in the number of levels of the IMC that should be treated (e.g., upper 3 or 4 levels versus all levels), the dose (e.g., 47.5 or 45 Gy to 95%) and the way to volume the region. Research from the Mayo Clinic mapped the location of 130 IMC metastases in 67 patients. The location was in the first three intercostal spaces in 102 of 130 nodal metastases (78%), whereas 18 of 130 IMNs (14%) were located caudal to the third intercostal space and 10 of 130 IMNs (8%) were located cranial to the first intercostal space. Of the 102 nodal metastases within the first three intercostal spaces, only 53% were located within the Radiation Therapy Oncology Group consensus volume. Ninety percent of lymph nodes within the first 3 intercostal spaces would have been encompassed within a 4-mm medial and lateral expansion on the IM vessels (89).
There have been significant advances in radiotherapy technology, with sophisticated imaging integrated into planning systems using techniques that protect the heart with shielding or deep inspiration breath holding, preferably using VMAT. VMAT can achieve highly conformal dose distributions by rotating the linear accelerator gantry at varying speeds through one or more arcs, while simultaneously changing the field shape. This allows shaping or sculpting radiation doses to complex cancer volumes while using modern equipment with on-board CT scans, with treatment times of about five minutes, to reduce the dose to normal structures such as the heart. These advances are likely to further improve the incremental benefit of radiation over and above surgery and systemic therapy and thus increase survival rates.
Evolution of radiation techniques
With the meta-analysis shown in Table 2 and the trials of IMC irradiation showing a benefit, there has been an increasing trend to irradiate the IMC, which is not normally dissected when the sentinel node drains to that location. Concern for irradiation of the heart stemmed back to 1974 when Stjernsward tabulated the survival rates of the six existing post-mastectomy trials at that time and found that radiation increased mortality in all trials by between 1% and 10% (90). He went on to state that “If the routine use of prophylactic local radiotherapy after radical mastectomy were stopped, survival might increase and resources might be saved”. Although Stjernsward was highly criticised at the time, the high-profile Darby publication found an association between higher mean heart dose and cardiac events (91). This case-control study noted that there is no safe dose of radiation to the heart and that there was a 7.4% increase in relative risk for each 1 Gy of mean heart dose. However, despite the important strengths of this study, there were also many weaknesses. The study involved patients from Sweden or Denmark treated between 1958 and 2001 before the advent of CT-based 3D planning. Patients in that era also received deep X-ray and some patients would have received larger fraction sizes. Patients in the “cases” group had a higher incidence of diabetes and chronic obstructive pulmonary disease, which implies other potential confounding risks of heart disease apart from radiation. Mean heart doses were derived from a selection of 20 cases. Further, relative risks are always magnified when communicating to patients. Supplementary Table S12 of the Darby publication shows the absolute risk of ischemic heart disease (IHD) by age of irradiation to the age of 80 years. If mean dose can be restricted to 4 Gy or less, the absolute risk is about 0.5% or 5 per 1,000 by age 80, irrespective of age at the time of treatment (91).
A second study validated the Darby data in a cohort of 910 consecutive female patients treated with breast-conserving radiation. The primary endpoint was cumulative incidence of acute coronary events (ACE’s) within 9 years of follow-up. Both mean heart dose and various dose-distribution parameters of the cardiac substructures were collected from three-dimensional computed tomography planning data. The cumulative incidence of ACE increased by 16.5% per Gy (P=0.042). Analysis showed that the volume of the left ventricle receiving 5 Gy (LV-V5) was the most important prognostic dose-volume parameter. (92).
Quantitative Analyses of Normal Tissue Effects in the Clinic recommends V25 <10%, and National Surgical Adjuvant Breast and Bowel Project (NSABP) B51 recommends a mean heart dose of ≤4 Gy mean. However, Figure 2C shows the damage that can occur to the coronary artery if irradiated. Such a patient may still have a mean heart dose of less than 4–5 Gy, but still receive high-dose radiation to the left anterior descending coronary artery (LAD). Figure 6 shows the impact that deep-inspiration breath-hold technique can have on reducing heart dose.
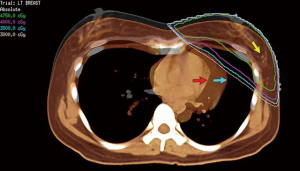
The impact of various techniques on the mean heart dose and other organs at risk is shown in Table 5 for a patient treated for breast cancer in the left breast using 6 Mv photons to 50 Gy in 25 fractions with either a synchronous boost using VMAT (57 Gy in 25 fractions) or a sequential boost (10 Gy in 5 fractions using 6 Mv photons or 15 Mev electrons) with the full IMC treated. The patient’s imaging is shown in Figure 3 and boost or lumpectomy PTV is shown in Figure 5. Treatment plans were generated for an Elekta Synergy accelerator (IntegrityTM Rel.1.1; MLCi2 with 0.5 cm leaf width) using the collapsed cone algorithm of Pinnacle3 (V9.10, Philips Radiation Oncology Systems, Fitchburg, WI, USA). The plan consisted of two rotational tangential arcs from 308° to 136° and back again. SmartArc plan optimisation was performed for gantry spacing at 4° during 80 iterations.
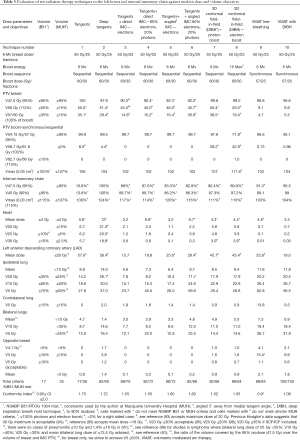
Full table
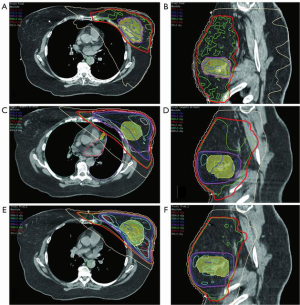
Table 5 shows the evolution of radiation techniques used in the late 1970’s and 80’s (techniques 1 & 2), through the 1990’s (techniques 3–5) and the 2000’s (techniques 6–10). Excluding tangential fields that did not target the IMC, the best plan that met all dose parameters was plan 10, the VMAT plan with deep inspiration breath hold (DIBH) (100% criteria met) (Figure 7A,B) and the worst plan was plan 2 (“deep tangents”) where 60% of criteria were met (Figure 7C,D). Of note, the 3D conformal field-in-field plan had lower dose homogeneity and higher doses to the coronary artery with lower doses to the IMC (Figure 7E,F). DIBH is a technique where radiation is only delivered when the patient takes a deep breath and holds it for up to 20 seconds at a time. DIBH not only keeps the patient still, but also creates a bigger space between the heart and the radiation beam allowing reduced heart and slightly reduced lung doses (Table 5, plans 9 and 10 and Figure 6).
The low-dose “bath” typical of VMAT or IMRT techniques has been raised as a concern but there is increasing evidence that the theoretical risk of second radiation-induced cancers is not increased by these more modern techniques (99) and is outweighed by improvements in local disease control and reduced cardiac toxicity (100). Our priority is generally PTV of boost > heart/LAD > lung > PTV breast/chest wall > supraclavicular/infraclavicular (SCF/ICF) fossa > IMC > contralateral breast similar to Nielsen et al. (93). The IMC will increase in priority for medial quadrant tumors and/or a PET-CT scan shows disease in an IMC node. Treating the IMC or other regional node basins increases the lung doses. Radiation pneumonitis is rare provided the mean ipsilateral lung dose is less than 18 Gy and V20 <35% (93). Lower thresholds for the lung are used by the author (Table 5).
A population-based study examined 10-year cause-specific actuarial mortality (breast cancer, cardiac, other cancers and other causes) for 1,242 patients with unilateral stage I–III invasive breast cancer in NSW, Australia, diagnosed over a 6-month period in 1995. For patients who received left-sided, right-sided or no RT, mortality due to breast cancer or other cancers was not significantly different (P=0.30 and P=0.11) between the three subgroups. Mortality due to cardiac and other causes was higher in patients who did not have radiotherapy (P=0.001 and P<0.001). A total of 52 cardiac deaths in 1,242 patients (4.2%) occurred: six of 274 patients (2.2%) in the left-sided radiotherapy group, four of 245 patients (1.6%) in the right-sided radiotherapy group (P=0.63) and 42 of 723 patients (5.8%) in the no radiotherapy group. Most cardiac deaths (46 of 52 cases) occurred in patients aged 70 years or older at the time of diagnosis. There were no differences in cardiac mortality between the three treatment groups for those aged 70 years or older (P=0.22, log-rank test), suggesting that the higher overall cardiac mortality rate in the no-RT group is due to a higher percentage of patients aged 70 years or older. Of the 10 patients who died from cardiac causes and had received RT, none had received chemotherapy or irradiation to the internal mammary chain (101). Nevertheless, with modern radiotherapy techniques and equipment (Figure 8), it remains critically important to do no harm and keep the lung and heart dose to acceptable tolerances as shown in Table 5, while never underestimating the substantial benefit of reduced breast cancer mortality from comprehensive radiation. Further survivorship programs incorporating wellness and heart health are important for the increasing cohort of survivors after a diagnosis of breast cancer.
Acknowledgements
The authors thank the following people and organizations that helped with the production of this manuscript: Philippa Sutton for editing and manuscript management, Genesis Cancer Care for Figures 5-8.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Grubbe EH. Priority in the Therapeutic Use of X-rays. Radiology 1933;21:156-62. [Crossref]
- Halsted WS. I. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann Surg 1907;46:1-19. [Crossref] [PubMed]
- Keynes G. The radium treatment of carcinoma of the breast. Br J Surg 1932;19:415-80. [Crossref]
- Ginzton EL, Nunan CS. History of microwave electron linear accelerators for radiotherapy. Int J Radiat Oncol Biol Phys 1985;11:205-16. [Crossref] [PubMed]
- Abdulkarim BS, Cuartero J, Hanson J, et al. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol 2011;29:2852-8. [Crossref] [PubMed]
- Lowery AJ, Kell MR, Glynn RW, et al. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat 2012;133:831-41. [Crossref] [PubMed]
- Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 2010;28:1684-91. [Crossref] [PubMed]
- Sjostrom M, Lundstedt D, Hartman L, et al. Response to Radiotherapy After Breast-Conserving Surgery in Different Breast Cancer Subtypes in the Swedish Breast Cancer Group 91 Radiotherapy Randomized Clinical Trial. J Clin Oncol 2017;35:3222-9. [Crossref] [PubMed]
- Horton JK, Jagsi R, Woodward WA, et al. Breast Cancer Biology: Clinical Implications for Breast Radiation Therapy. Int J Radiat Oncol Biol Phys 2018;100:23-37. [Crossref] [PubMed]
- Taylor R, Stubbs JM, Langlands AO, et al. Predictors of mastectomy for women with breast cancer in the greater western region of sydney. Breast J 1999;5:116-21. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143-50. [Crossref] [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer 2003;98:697-702. [Crossref] [PubMed]
- Arriagada R, Le MG, Rochard F, et al. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol 1996;14:1558-64. [Crossref] [PubMed]
- Blichert-Toft M, Nielsen M, During M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol 2008;47:672-81. [Crossref] [PubMed]
- Boyages J, Harris JR. Local therapy of invasive disease. Hematol Oncol Clin North Am 1989;3:675-90. [Crossref] [PubMed]
- Boyages J, Recht A, Connolly JL, et al. Early breast cancer: predictors of breast recurrence for patients treated with conservative surgery and radiation therapy. Radiother Oncol 1990;19:29-41. [Crossref] [PubMed]
- Leong C, Boyages J, Jayasinghe UW, et al. Effect of Margins on Ipsilateral Breast Tumor Recurrence after Breast Conservation Therapy for Lymph Node-Negative Breast Carcinoma. Cancer 2004;100:1823-32. [Crossref] [PubMed]
- Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer 2010;46:3219-32. [Crossref] [PubMed]
- Houssami N, Marinovich ML. Margins in Breast-Conserving Surgery for Early Breast Cancer: How Much is Good Enough? Current Breast Cancer Reports 2016;8:127-34. [Crossref]
- Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int J Radiat Oncol Biol Phys 2014;88:553-64. [Crossref] [PubMed]
- Bodilsen A, Bjerre K, Offersen BV, et al. Importance of margin width in breast-conserving treatment of early breast cancer. J Surg Oncol 2016;113:609-15. [Crossref] [PubMed]
- Gupta S, King WD, Korzeniowski M, et al. The Effect of Waiting Times for Postoperative Radiotherapy on Outcomes for Women Receiving Partial Mastectomy for Breast Cancer: a Systematic Review and Meta-Analysis. Clin Oncol (R Coll Radiol) 2016;28:739-49. [Crossref] [PubMed]
- Holland R, Connolly JL, Gelman R, et al. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J Clin Oncol 1990;8:113-8. [Crossref] [PubMed]
- Boyages J, Bosch C, Langlands AO, et al. Breast conservation: long-term Australian data. Int J Radiat Oncol Biol Phys 1992;24:253-60. [Crossref] [PubMed]
- Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 2015;16:47-56. [Crossref] [PubMed]
- Vrieling C, van Werkhoven E, Maingon P, et al. Prognostic Factors For Local Control in Breast Cancer After Long-term Follow-up in the EORTC Boost vs. No Boost Trial: A Randomized Clinical Trial. JAMA Oncol 2017;3:42-8. [Crossref] [PubMed]
- Mannino M, Yarnold JR. Local relapse rates are falling after breast conserving surgery and systemic therapy for early breast cancer: can radiotherapy ever be safely withheld? Radiother Oncol 2009;90:14-22. [Crossref] [PubMed]
- Hunt KK, Ballman KV, McCall LM, et al. Factors associated with local-regional recurrence after a negative sentinel node dissection: results of the ACOSOG Z0010 trial. Ann Surg 2012;256:428-36. [Crossref] [PubMed]
- Kindts I, Laenen A, Depuydt T, et al. Tumour bed boost radiotherapy for women after breast-conserving surgery. Cochrane Database Syst Rev 2017;11:Cd011987. [PubMed]
- Adams BJ, Zoon CK, Stevenson C, et al. The role of margin status and reexcision in local recurrence following breast conservation surgery. Ann Surg Oncol 2013;20:2250-5. [Crossref] [PubMed]
- Vos EL, Jager A, Verhoef C, et al. Overall survival in patients with a re-excision following breast conserving surgery compared to those without in a large population-based cohort. Eur J Cancer 2015;51:282-91. [Crossref] [PubMed]
- Boersma LJ, Janssen T, Elkhuizen PH, et al. Reducing interobserver variation of boost-CTV delineation in breast conserving radiation therapy using a pre-operative CT and delineation guidelines. Radiother Oncol 2012;103:178-82. [Crossref] [PubMed]
- Wang W, French J, Boyages J. Put the felt pen away: time to move on from a clinical mark-up for a breast boost. J Med Imaging Radiat Oncol 2012;56:375-8. [Crossref] [PubMed]
- Recht A, Harris JR. To boost or not to boost, and how to do it. Int J Radiat Oncol Biol Phys 1991;20:177-8. [Crossref] [PubMed]
- Li XA, Tai A, Arthur DW, et al. Variability of target and normal structure delineation for breast cancer radiotherapy: an RTOG Multi-Institutional and Multiobserver Study. Int J Radiat Oncol Biol Phys 2009;73:944-51. [Crossref] [PubMed]
- van Mourik AM, Elkhuizen PH, Minkema D, et al. Multiinstitutional study on target volume delineation variation in breast radiotherapy in the presence of guidelines. Radiother Oncol 2010;94:286-91. [Crossref] [PubMed]
- Vicini FA, Kestin LL, Goldstein NS. Defining the clinical target volume for patients with early-stage breast cancer treated with lumpectomy and accelerated partial breast irradiation: a pathologic analysis. Int J Radiat Oncol Biol Phys 2004;60:722-30. [Crossref] [PubMed]
- Bazan J, DiCostanzo D, Kuhn K, et al. Likelihood of unacceptable normal tissue doses in breast cancer patients undergoing regional nodal irradiation in routine clinical practice. Pract Radiat Oncol 2017;7:154-60. [Crossref] [PubMed]
- Inc NF. Standard or comprehensive radiation therapy in treating patients with early-stage breast cancer previously treated with chemotherapy and surgery. 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01872975. Accessed 3 December 2017.
- Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. [Crossref] [PubMed]
- Esposito E, Anninga B, Harris S, et al. Intraoperative radiotherapy in early breast cancer. Br J Surg 2015;102:599-610. [Crossref] [PubMed]
- Boyages J. Radiation therapy and early breast cancer: current controversies. Med J Aust 2017;207:216-22. [Crossref] [PubMed]
- Matuschek C, Bolke E, Haussmann J, et al. The benefit of adjuvant radiotherapy after breast conserving surgery in older patients with low risk breast cancer- a meta-analysis of randomized trials. Radiat Oncol 2017;12:60. [Crossref] [PubMed]
- Blamey RW, Bates T, Chetty U, et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer 2013;49:2294-302. [Crossref] [PubMed]
- Fyles AW, McCready DR, Manchul LA, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med 2004;351:963-70. [Crossref] [PubMed]
- Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med 2004;351:971-7. [Crossref] [PubMed]
- Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015;16:266-73. [Crossref] [PubMed]
- Potter R, Gnant M, Kwasny W, et al. Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer. Int J Radiat Oncol Biol Phys 2007;68:334-40. [Crossref] [PubMed]
- Winzer KJ, Sauer R, Sauerbrei W, et al. Radiation therapy after breast-conserving surgery; first results of a randomised clinical trial in patients with low risk of recurrence. Eur J Cancer 2004;40:998-1005. [Crossref] [PubMed]
- Killander F, Karlsson P, Anderson H, et al. No breast cancer subgroup can be spared postoperative radiotherapy after breast-conserving surgery. Fifteen-year results from the Swedish Breast Cancer Group randomised trial, SweBCG 91 RT. Eur J Cancer 2016;67:57-65. [Crossref] [PubMed]
- Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol 2006;7:467-71. [Crossref] [PubMed]
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086-94. [Crossref] [PubMed]
- Spooner D, Stocken DD, Jordan S, et al. A randomised controlled trial to evaluate both the role and the optimal fractionation of radiotherapy in the conservative management of early breast cancer. Clin Oncol (R Coll Radiol) 2012;24:697-706. [Crossref] [PubMed]
- Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513-20. [Crossref] [PubMed]
- Bane AL, Whelan TJ, Pond GR, et al. Tumor factors predictive of response to hypofractionated radiotherapy in a randomized trial following breast conserving therapy. Ann Oncol 2014;25:992-8. [Crossref] [PubMed]
- Hannan R, Thompson RF, Chen Y, et al. Hypofractionated whole-breast radiation therapy: does breast size matter? Int J Radiat Oncol Biol Phys 2012;84:894-901. [Crossref] [PubMed]
- Agrawal RK, Alhasso A, Barrett-Lee PJ, et al. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015). Radiother Oncol 2011;100:93-100. [Crossref] [PubMed]
- Valle LF, Agarwal S, Bickel KE, et al. Hypofractionated whole breast radiotherapy in breast conservation for early-stage breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res Treat 2017;162:409-17. [Crossref] [PubMed]
- Hickey BE, James ML, Lehman M, et al. Hypofractionated radiation therapy for early breast cancer. Cochrane Database Syst Rev 2016. [Crossref] [PubMed]
- Clinical Practice Recommendations and Practice Points. Australian Government Cancer Australia. 2016. Available online: https://canceraustralia.gov.au/publications-and-resources/clinical-practice-guidelines/hypofractionated-radiotherapy-early-operable-breast-cancer/clinical-practice-recommendations-and-practice-points. Accessed 7/10/2016.
- Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;81:59-68. [Crossref] [PubMed]
- Harnett A, Smallwood J, Titshall V, et al. Diagnosis and treatment of early breast cancer, including locally advanced disease--summary of NICE guidance. BMJ 2009;338:598-600. [Crossref] [PubMed]
- Dellas K, Vonthein R, Zimmer J, et al. Hypofractionation with simultaneous integrated boost for early breast cancer: results of the German multicenter phase II trial (ARO-2010-01). Strahlenther Onkol 2014;190:646-53. [Crossref] [PubMed]
- Skowronek J, Wawrzyniak-Hojczyk M, Ambrochowicz K. Brachytherapy in accelerated partial breast irradiation (APBI) – review of treatment methods. Journal of Contemporary Brachytherapy 2012;4:152-64. [Crossref] [PubMed]
- Akhtari M, Teh BS. Accelerated partial breast irradiation: advances and controversies. Chinese Journal of Cancer 2016;35:31. [Crossref] [PubMed]
- Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet 2016;387:229-38. [Crossref] [PubMed]
- Polgar C, Ott OJ, Hildebrandt G, et al. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year results of a randomised, controlled, phase 3 trial. Lancet Oncol 2017;18:259-68. [Crossref] [PubMed]
- Livi L, Meattini I, Marrazzo L, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer 2015;51:451-63. [Crossref] [PubMed]
- Rodriguez N, Sanz X, Dengra J, et al. Five-year outcomes, cosmesis, and toxicity with 3-dimensional conformal external beam radiation therapy to deliver accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys 2013;87:1051-7. [Crossref] [PubMed]
- Coles CE, Griffin CL, Kirby AM, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017;390:1048-60. [Crossref] [PubMed]
- Gentilini O, Botteri E, Leonardi MC, et al. Ipsilateral axillary recurrence after breast conservative surgery: The protective effect of whole breast radiotherapy. Radiother Oncol 2017;122:37-44. [Crossref] [PubMed]
- Correa C, Harris EE, Leonardi MC, et al. Accelerated Partial Breast Irradiation: Executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract Radiat Oncol 2017;7:73-9. [Crossref] [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [Crossref] [PubMed]
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. [Crossref] [PubMed]
- Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91-102. [Crossref] [PubMed]
- Kaidar-Person O, Poortmans P, Klimberg S, et al. Haste makes waste: are the data regarding TARGIT-A IORT ready for prime time? Breast Cancer Res Treat 2014;147:221-2. [Crossref] [PubMed]
- Cuzick J. Radiotherapy for breast cancer, the TARGIT-A trial. Lancet 2014;383:1716. [Crossref] [PubMed]
- Hepel J, Wazer DE. A flawed study should not define a new standard of care. Int J Radiat Oncol Biol Phys 2015;91:255-7. [Crossref] [PubMed]
- (NICE) NIfHaCE. Intrabeam radiotherapy system for adjuvant treatment of early breast cancer. 2018. Available online: https://www.nice.org.uk/guidance/ta501/chapter/Recommendations. Accessed 16/3/2018 2018.
- McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. [Crossref] [PubMed]
- Chang JS, Park W, Kim YB, et al. Long-term survival outcomes following internal mammary node irradiation in stage II-III breast cancer: results of a large retrospective study with 12-year follow-up. Int J Radiat Oncol Biol Phys 2013;86:867-72. [Crossref] [PubMed]
- Courdi A, Chamorey E, Ferrero JM, et al. Influence of internal mammary node irradiation on long-term outcome and contralateral breast cancer incidence in node-negative breast cancer patients. Radiother Oncol 2013;108:259-65. [Crossref] [PubMed]
- Hennequin C, Bossard N, Servagi-Vernat S, et al. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat Oncol Biol Phys 2013;86:860-6. [Crossref] [PubMed]
- Thorsen LB, Offersen BV, Dano H, et al. DBCG-IMN: A Population-Based Cohort Study on the Effect of Internal Mammary Node Irradiation in Early Node-Positive Breast Cancer. J Clin Oncol 2016;34:314-20. [Crossref] [PubMed]
- Verma V, Vicini F, Tendulkar RD, et al. Role of Internal Mammary Node Radiation as a Part of Modern Breast Cancer Radiation Therapy: A Systematic Review. Int J Radiat Oncol Biol Phys 2016;95:617-31. [Crossref] [PubMed]
- Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med 2015;373:307-16. [Crossref] [PubMed]
- Jethwa KR, Kahila MM, Hunt KN, et al. Delineation of Internal Mammary Nodal Target Volumes in Breast Cancer Radiation Therapy. Int J Radiat Oncol Biol Phys 2017;97:762-9. [Crossref] [PubMed]
- Stjernsward J. Decreased survival related to irradiation postoperatively in early operable breast cancer. Lancet 1974;2:1285-6. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- van den Bogaard VA, Ta BD, van der Schaaf A, et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients With Breast Cancer Treated With Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J Clin Oncol 2017;35:1171-8. [Crossref] [PubMed]
- Nielsen MH, Berg M, Pedersen AN, et al. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: national guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol 2013;52:703-10. [Crossref] [PubMed]
- Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose–volume effects in the heart. Int J Radiat Oncol Biol Phys 2010;76:S77-85. [Crossref] [PubMed]
- Lind PA, Wennberg B, Gagliardi G, et al. Pulmonary complications following different radiotherapy techniques for breast cancer, and the association to irradiated lung volume and dose. Breast Cancer Res Treat 2001;68:199-210. [Crossref] [PubMed]
- Popescu CC, Olivotto IA, Beckham WA, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys 2010;76:287-95. [Crossref] [PubMed]
- Kwa SL, Lebesque JV, Theuws JC, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys 1998;42:1-9. [Crossref] [PubMed]
- Pinnix CC, Smith GL, Milgrom S, et al. Predictors of radiation pneumonitis in patients receiving intensity modulated radiation therapy for Hodgkin and non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2015;92:175-82. [Crossref] [PubMed]
- Filippi AR, Vanoni V, Meduri B, et al. Intensity Modulated Radiation Therapy and Second Cancer Risk in Adults. Int J Radiat Oncol Biol Phys 2018;100:17-20. [Crossref] [PubMed]
- Donovan EM, James H, Bonora M, et al. Second cancer incidence risk estimates using BEIR VII models for standard and complex external beam radiotherapy for early breast cancer. Med Phys 2012;39:5814-24. [Crossref] [PubMed]
- Wang W, O'Connell D, Stuart K, et al. Analysis of 10-year cause-specific mortality of patients with breast cancer treated in New South Wales in 1995. J Med Imaging Radiat Oncol 2011;55:516-25. [Crossref] [PubMed]