
The use of acellular dermal matrix in breast reconstruction: evolution of techniques over 2 decades
Introduction
Acellular dermal matrices (ADMs) are human-, bovine-, or porcine-derived biotechnologically engineered tissues that have served a myriad of purposes across surgical subspecialties. Tissue processing removes the cellular antigens capable of producing an immunologic response while maintaining the structural matrix that encourages angiogenesis and tissue regeneration. The initial reported clinical use of ADMs was in the management of full-thickness burns in 1995 (1), and they subsequently have had a number of applications within plastic and reconstructive surgery, including in abdominal hernia repair (2), rhinoplasty (3), facial soft tissue augmentation (4,5), lip augmentation (6), and oculofacial procedures (7).
After the first usage in the breast by the senior author in 2001, ADMs have become a cornerstone of implant-based immediate breast reconstruction over the last 2 decades. Immediate breast reconstruction became preferred to delayed reconstruction in the 1990’s (8) due to its improved psychosocial morbidity (9,10), decreased cost (11), and optimal cosmetic outcomes facilitated by the advent of skin-sparing mastectomies (12). Prior to the use of ADMs, effective immediate prosthesis-based breast reconstruction necessitated the use of a submuscular expander with full muscle coverage to improve soft tissue coverage prior to a permanent implant placement or removal of the filling port attached to a combination device (13). The disadvantages of this approach include infectious complications requiring multiple operations (14) and patient discomfort (15).
Dual-plane subpectoral approach
In addition to the transition from delayed to immediate breast reconstruction, the location of implant placement also greatly evolved toward the end of the 20th century from a fully submuscular approach to partial muscular coverage of the implant, also known as the dual-plane subpectoral approach. While the latter approach shortened operative time and facilitated better expansion of the lower pole, this required “marionette” sutures to the inframammary fold (IMF) for definition of the IMF and lateral mammary fold (LMF) (16,17). Furthermore, the lack of implant support allowed for implant migration, malposition, and exposure due to the absence of lower pole support and inadequate inferolateral soft tissue coverage (18).
ADMs were initially used to decrease the visible rippling of breast implants in patients with thin soft tissue coverage. Then in 2001, the senior author pioneered the use of ADMs as soft tissue coverage and structural support for prosthetic-based immediate breast reconstruction (19). The technique used involves the creation of the subpectoral pocket followed by inferomedial elevation and precise division of the pectoralis major muscle from the inferior margin. The ADM is then sutured to the inferior pectoralis major muscle extending to the LMF with no elevation of the serratus anterior muscle. The implant is then inserted beneath the pectoralis-ADM layer, and the ADM is sutured to the IMF to completely cover and secure the implant with a hand-in-glove fit. Subcutaneous and subpectoral suction drains are then placed followed by skin closure (20). Outcomes for this approach have been reported for up to 8–13 years postoperatively, and include good cosmetic results (Figure 1) with a low incidence of capsular contracture (0.4%) and low overall complication rate (3.9–8.6%), which is comparable to the published complication rates for two-stage breast reconstruction. Complications included skin necrosis, infection, implant loss, seroma, and hematoma (21,22). The development of complications was predicted by older age, smoking, non-nipple sparing mastectomy, and larger implant size (21).

Many surgeons developed similar techniques for using ADMs in implant or two stage expander-based dual-plane reconstruction with low complication rates and good aesthetic outcomes (23-28). One difference in the technique described by Breuing and Warren [2005] is the order of ADM attachment to the pectoralis major muscle and to the chest wall at the IMF and LMF. While the technique described above advocates for initial ADM attachment to the pectoralis major after the creation of the subpectoral pocket, this technique involves first suturing the ADM to the chest wall at the IMF with extension to the LMF prior to temporary implant sizer placement to ensure complete implant coverage. Closed-suction drains are then placed, followed by exchange of the sizer with a permanent silicone implant and attachment of the ADM to the pectoralis major muscle to close the implant pocket (27). Namnoum [2009] and Glasberg and Light [2012] reported similar techniques for tissue expander placement (29,30). Likewise, Spear et al. [2008] describes a technique for immediate breast reconstruction with tissue expanders that involves anchoring the ADM to the chest wall along the preoperatively determined IMF and LMF with many untied interrupted sutures or a running suture that is not initially tightly secured in order to allow for later tension adjustments. This is followed by seating of the tissue expander, suturing of the ADM to the pectoralis major muscle, and finally, securing the sutures at the IMF and LMF (25).
Topol et al. [2008] further expanded the dual-plane approach by using ADMs in immediate implant-based reconstruction in conjunction with lower chest advancement flaps and IMF reconstructions in order to decrease the risk of a vertically lifted breast and enhance symmetry in unilateral breast reconstruction. IMF reconstruction with or without LMF reconstruction was done prior to pectoralis muscle elevation. A sheet of extra thick ADM was subsequently sutured to the inferior breast skin flap 1 cm above the IMF extending to the underside of the lateral skin at or just above the LMF. The implant was then seated against the IMF and the inferior aspect of the pectoralis major muscle was sutured to the superior aspect of the ADM (31).
Breuing and Colwell [2007], who termed the technique “The AlloDerm hammock,” broadened the use of the partial subpectoral approach to include delayed reconstruction, reconstruction for congenital breast aplasia, and expander-implant reconstruction (32). Chepla et al. [2012] then introduced the “partial AlloDerm sling” with the aim of possibly providing adequate expander support while reducing costs associated with ADM use. The authors employed this technique in all patients with a caudal insertion of the pectoralis major muscle <1 cm from IMF. In these patients, instead of releasing the pectoralis from the point of its medial origin to create the partial submuscular expander pocket, the aspects of the pectoralis muscle lying medially and inferiorly at the IMF were left intact. The smallest size of ADM possible was then used to cover the exposed lateral aspect of the submuscular pocket. Using this technique, the authors were able to use less than 64 cm2 of ADM in 20% of the reconstructed breasts included in this study, which is 20 cm2 less than the reported average use of ADM per breast in the literature (33). Hadad et al. [2015] used a similar ADM-sparing technique with ADM use limited to the lateral aspect of the subpectoral pocket. They achieved good aesthetic results with a decrease in seroma, infection, and rate of reconstruction loss as compared to their non-ADM sparing technique (34).
Maxwell and Gabriel [2016] created an integrated approach termed the “bioengineered breast concept,” which described the use of ADMs in two-stage breast reconstruction for support of shaped textured form-stable implants, followed by later fat grafting for enhanced implant coverage. First, an expander is placed subpectorally with ADM used for inferior coverage. During the second stage of reconstruction when the permanent implant is substituted for the expander, a capsulotomy is performed and ADM is sutured at the upper pole of the breast pocket subpectorally followed by overlying subcutaneous autologous fat grafting in patients with inadequate soft tissue thickness or low body mass index (BMI) (35).
Prepectoral breast reconstruction with ADM
While cosmetic outcomes of the ADM-assisted dual-plane subpectoral approach have generally been quite good with limited postoperative complications, the risk of postoperative pain and animation deformity with pectoralis contraction or spasm remains a potential consequence that can significantly affect quality of life (36,37). This disadvantage of the dual-plane subpectoral approach has fueled the search for a less invasive method of reconstruction, leading many to return to the prepectoral approach once popularized in the 1970’s (38,39). At that time, this involved placement of an implant or expander in the prepectoral subcutaneous space, which previously resulted in a high risk of infection, hematoma, capsular contracture, implant malpositioning, and implant extrusion (40-42). In contrast to that initial approach, the recent movement to return to prepectoral breast reconstruction involves the use of ADMs to provide implant support and soft tissue coverage in order to avoid the complications that were experienced.
While some surgeons began returning to the prepectoral approach as a revisionary operation to correct animation deformity (43), others began to use the approach in primary reconstructive operations. Reitsamer and Peintinger [2015] described porcine ADM use with shaped silicone gel-filled implants in primary prepectoral breast reconstruction following nipple-sparing mastectomy by employing a technique that involved extracorporeal suturing, trimming, and incisions of two sheets of ADM in order to create a fitted ADM envelope with angular ADM flaps that could be used to secure the implant in the prepectoral space (44). Additional reported techniques have involved suturing the anteriorly placed ADM to the periphery of the implant pocket, while other techniques involve partially or completely wrapping the implant with ADM secured with sutures either circumferentially, in a centromedial pattern on the posterior surface of the implant, or in a manufacture designed pattern prior to implant placement (45-51). Others have created a compound implant pocket made with an inferior dermal flap that is attached at its superior border to ADM, which then serves to cover the remainder of the implant (52). Furthermore, ADM has been used as anterior augmentation and inferolateral support of a Vicryl mesh implant pocket in patients at a high risk of implant rippling or those likely to undergo subsequent fat grafting (53,54).
Nahabedian [2018] notes that while many of the techniques described above have entered current practice, the U.S. Food and Drug Administration has indicated that on-label techniques include only those in which the ADM is used for tissue support rather than implant support. As such, on-label techniques include those where the ADM is sutured to the periphery of the implant pocket followed by implant placement, while off-label techniques include those where the ADM is secured to the implant prior to prepectoral placement of the implant-ADM construct (55).
Furthermore, the bioengineered breast concept that had previously been reported in two-stage partial subpectoral breast reconstruction was applied to a prepectoral approach, which similar to its initial application, involved the use of ADM to support a cohesive gel implant with later overlying autologous fat grafting. As is noted with most prepectoral reconstructions, this approach necessitates the presence of a well-vascularized skin flap due to its proximity to the implant, and as such, is contraindicated in patients with prior breast irradiation and active smoking. Contraindications to this approach also include patients with advanced malignancy, proximal tumors, and high risk of cancer recurrence (56).
Sbitany et al. [2017] compared the dual-plane submuscular approach to the prepectoral approach in expander-based breast reconstruction following nipple-sparing mastectomy and found that while the latter approach decreased expander migration in patients undergoing post-mastectomy radiation therapy, there were no other significant differences in rates of major complications. Patients were chosen by the primary author to undergo the prepectoral approach based on clinical considerations including viability of mastectomy skin flap, size and ptosis of the breast skin envelope, and oncologic setting. The surgical technique involves first suturing the ADM anterior to the pectoralis major muscle 3 cm above the planned IMF. The ADM is then brought down to the inferior pole where it is folded back up at the IMF and subsequently sutured in place to the chest wall to create an ADM cuff that provides further expander support. The expander is then placed and the ADM is pulled up and sutured in place at its medial, superior, and lateral borders such that it covers the anterior aspect of the expander. After a mean follow-up time of approximately 1-year, the author reported good aesthetic outcomes with no significant differences in complication rates, aside from that mentioned above, in those patients who underwent the prepectoral approach as compared to those who underwent the dual-plane approach (57).
However, despite encouraging early outcomes of the prepectoral approach, some cite the expense of ADM use as a limitation to the prepectoral approach, where a substantial amount of material is used to allow for adequate implant coverage (58). Others argue that long-term economic benefits may lie in superior outcomes that include fewer secondary operations, decreased capsular contracture, and decreased narcotic use secondary to improved postoperative pain (47,58). Nevertheless in order to avoid this concern, some have sought to decrease ADM use through the use of shaped and fenestrated ADMs for anterior coverage of prepectoral implants, which are made as parallel rows of staggered longitudinal cuts that are perpendicular to a posteroanterior line originating at the chest wall (59). This approach allows the sheet to expand, thus potentially decrease the amount of ADM used, with preliminary results showing comparable complication rates to others performing ADM-assisted prepectoral reconstruction (60).
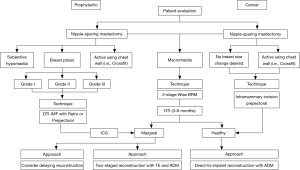
The opinion of the senior author of this paper concludes that are many ways to achieve an excellent outcome and immediate breast reconstruction. The options of implant-based reconstruction have been evaluated and the type of reconstruction should be chosen for each patient individually (Figure 2). The retropectoral ADM approach is excellent for most patients but in those patients who are very active with her pectoralis muscle, professional athletes, or those who have a great desire for shaped implants a prepectoral approach makes sense. The prepectoral approach seems to necessitate a secondary procedure with subcutaneous fat grafting and many patients would like a one-stage approach. Animation deformity occurs in many patients in which it is not bothersome but in others it is very difficult and switching from the sub-pectoral to the subcutaneous position has been done on many occasions with full ADM coverage.
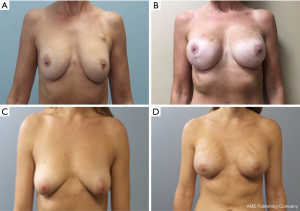
There is obvious rippling and wrinkling in many patients who have thin upper pole skin with the prepectoral approach (Figure 3), which influences the choices given to the patient on which approach would best be suited for them individually.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 1995;21:243-8. [Crossref] [PubMed]
- Buinewicz B, Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg 2004;52:188-94. [Crossref] [PubMed]
- Sherris DA, Oriel BS. Human acellular dermal matrix grafts for rhinoplasty. Aesthet Surg J 2011;31:95S-100S. [Crossref] [PubMed]
- Terino EO. Alloderm acellular dermal graft: applications in aesthetic soft-tissue augmentation. Clin Plast Surg 2001;28:83-99. [PubMed]
- Achauer BM, VanderKam VM, Celikoz B, et al. Augmentation of facial soft-tissue defects with Alloderm dermal graft. Ann Plast Surg 1998;41:503-7. [Crossref] [PubMed]
- Rohrich RJ, Reagan BJ, Adams WP Jr, et al. Early results of vermilion lip augmentation using acellular allogeneic dermis: an adjunct in facial rejuvenation. Plast Reconstr Surg 2000;105:409-16; discussion 417-8. [Crossref] [PubMed]
- Bee YS, Alonzo B, Ng JD. Review of AlloDerm Acellular Human Dermis Regenerative Tissue Matrix in Multiple Types of Oculofacial Plastic and Reconstructive Surgery. Ophthalmic Plast Reconstr Surg 2015;31:348-51. [Crossref] [PubMed]
- Losken A, Carlson GW, Bostwick J 3rd, et al. Trends in unilateral breast reconstruction and management of the contralateral breast: the Emory experience. Plast Reconstr Surg 2002;110:89-97. [Crossref] [PubMed]
- Stevens LA, McGrath MH, Druss RG, et al. The psychological impact of immediate breast reconstruction for women with early breast cancer. Plast Reconstr Surg 1984;73:619-28. [Crossref] [PubMed]
- Dean C, Chetty U, Forrest AP. Effects of immediate breast reconstruction on psychosocial morbidity after mastectomy. Lancet 1983;1:459-62. [Crossref] [PubMed]
- Khoo A, Kroll SS, Reece GP, et al. A comparison of resource costs of immediate and delayed breast reconstruction. Plast Reconstr Surg 1998;101:964-8; discussion 969-70. [Crossref] [PubMed]
- Singletary SE, Kroll SS. Skin-sparing mastectomy with immediate breast reconstruction. Adv Surg 1996;30:39-52. [PubMed]
- Ward J, Cohen IK, Knaysi GA, et al. Immediate breast reconstruction with tissue expansion. Plast Reconstr Surg 1987;80:559-66. [Crossref] [PubMed]
- Sue GR, Sun BJ, Lee GK. Complications After Two-Stage Expander Implant Breast Reconstruction Requiring Reoperation: A Critical Analysis of Outcomes. Ann Plast Surg 2018;80:S292-4. [PubMed]
- Lo KK, Aycock JK. A blinded randomized controlled trial to evaluate the use of botulinum toxin for pain control in breast reconstruction with tissue expanders. Ann Plast Surg 2015;74:281-3. [Crossref] [PubMed]
- Serra-Renom JM, Fontdevila J, Monner J, et al. Mammary reconstruction using tissue expander and partial detachment of the pectoralis major muscle to expand the lower breast quadrants. Ann Plast Surg 2004;53:317-21. [Crossref] [PubMed]
- Spear SL, Pelletiere CV. Immediate breast reconstruction in two stages using textured, integrated-valve tissue expanders and breast implants. Plast Reconstr Surg 2004;113:2098-103. [Crossref] [PubMed]
- Nahabedian MY, Spear SL. Acellular dermal matrix for secondary procedures following prosthetic breast reconstruction. Aesthet Surg J 2011;31:38S-50S. [Crossref] [PubMed]
- Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg 2006;57:1-5. [Crossref] [PubMed]
- Salzberg CA. Focus on technique: one-stage implant-based breast reconstruction. Plast Reconstr Surg 2012;130:95S-103S. [Crossref] [PubMed]
- Hunsicker LM, Ashikari AY, Berry C, et al. Short-Term Complications Associated With Acellular Dermal Matrix-Assisted Direct-to-Implant Breast Reconstruction. Ann Plast Surg 2017;78:35-40. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24. [Crossref] [PubMed]
- Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg 2007;120:373-81. [Crossref] [PubMed]
- Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg 2009;124:1735-40. [Crossref] [PubMed]
- Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg 2008;32:418-25. [Crossref] [PubMed]
- Bindingnavele V, Gaon M, Ota KS, et al. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg 2007;60:1214-8. [Crossref] [PubMed]
- Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9. [Crossref] [PubMed]
- Gamboa-Bobadilla GM. Implant breast reconstruction using acellular dermal matrix. Ann Plast Surg 2006;56:22-5. [Crossref] [PubMed]
- Namnoum JD. Expander/implant reconstruction with AlloDerm: recent experience. Plast Reconstr Surg 2009;124:387-94. [Crossref] [PubMed]
- Glasberg SB, Light D. AlloDerm and Strattice in breast reconstruction: a comparison and techniques for optimizing outcomes. Plast Reconstr Surg 2012;129:1223-33. [Crossref] [PubMed]
- Topol BM, Dalton EF, Ponn T, et al. Immediate single-stage breast reconstruction using implants and human acellular dermal tissue matrix with adjustment of the lower pole of the breast to reduce unwanted lift. Ann Plast Surg 2008;61:494-9. [Crossref] [PubMed]
- Breuing KH, Colwell AS. Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg 2007;59:250-5. [Crossref] [PubMed]
- Chepla KJ, Dagget JR, Soltanian HT. The partial AlloDerm sling: reducing allograft costs associated with breast reconstruction. J Plast Reconstr Aesthet Surg 2012;65:924-30. [Crossref] [PubMed]
- Hadad I, Liu AS, Guo L. A New Approach to Minimize Acellular Dermal Matrix Use in Prosthesis-based Breast Reconstruction. Plast Reconstr Surg Glob Open 2015;3:e472. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. Bioengineered Breast: Concept, Technique, and Preliminary Results. Plast Reconstr Surg 2016;137:415-21. [Crossref] [PubMed]
- Becker H, Fregosi N. The Impact of Animation Deformity on Quality of Life in Post-Mastectomy Reconstruction Patients. Aesthet Surg J 2017;37:531-6. [Crossref] [PubMed]
- Walia GS, Aston J, Bello R, et al. Prepectoral Versus Subpectoral Tissue Expander Placement: A Clinical and Quality of Life Outcomes Study. Plast Reconstr Surg Glob Open 2018;6:e1731. [Crossref] [PubMed]
- Snyderman RK, Guthrie RH. Reconstruction of the female breast following radical mastectomy. Plast Reconstr Surg 1971;47:565-7. [Crossref] [PubMed]
- Birnbaum L, Olsen JA. Breast reconstruction following radical mastectomy, using custom designed implants. Plast Reconstr Surg 1978;61:355-63. [Crossref] [PubMed]
- Gruber RP, Kahn RA, Lash H, et al. Breast reconstruction following mastectomy: a comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg 1981;67:312-7. [Crossref] [PubMed]
- Redfern AB, Hoopes JE. Subcutaneous mastectomy: a plea for conservatism. Plast Reconstr Surg 1978;62:706-7. [Crossref] [PubMed]
- Schlenker JD, Bueno RA, Ricketson G, et al. Loss of silicone implants after subcutaneous mastectomy and reconstruction. Plast Reconstr Surg 1978;62:853-61. [Crossref] [PubMed]
- Hammond DC, Schmitt WP, O'Connor EA. Treatment of breast animation deformity in implant-based reconstruction with pocket change to the subcutaneous position. Plast Reconstr Surg 2015;135:1540-4. [Crossref] [PubMed]
- Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg 2015;68:162-7. [Crossref] [PubMed]
- Becker H, Lind JG 2nd, Hopkins EG. Immediate Implant-based Prepectoral Breast Reconstruction Using a Vertical Incision. Plast Reconstr Surg Glob Open 2015;3:e412. [Crossref] [PubMed]
- Downs RK, Hedges K. An Alternative Technique for Immediate Direct-to-Implant Breast Reconstruction-A Case Series. Plast Reconstr Surg Glob Open 2016;4:e821. [Crossref] [PubMed]
- Cattelani L, Polotto S, Arcuri MF, et al. One-Step Prepectoral Breast Reconstruction With Dermal Matrix-Covered Implant Compared to Submuscular Implantation: Functional and Cost Evaluation. Clin Breast Cancer 2018;18:e703-11. [Crossref] [PubMed]
- Berna G, Cawthorn SJ, Papaccio G, et al. Evaluation of a novel breast reconstruction technique using the Braxon® acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg 2017;87:493-8. [Crossref] [PubMed]
- Woo A, Harless C, Jacobson SR. Revisiting an Old Place: Single-Surgeon Experience on Post-Mastectomy Subcutaneous Implant-Based Breast Reconstruction. Breast J 2017;23:545-53. [Crossref] [PubMed]
- Vidya R, Cawthorn SJ. Muscle-Sparing ADM-Assisted Breast Reconstruction Technique Using Complete Breast Implant Coverage: A Dual-Institute UK-Based Experience. Breast Care (Basel) 2017;12:251-4. [Crossref] [PubMed]
- Sigalove S. Options in Acellular Dermal Matrix-Device Assembly. Plast Reconstr Surg 2017;140:39S-42S. [Crossref] [PubMed]
- Caputo GG, Marchetti A, Dalla Pozza E, et al. Skin-Reduction Breast Reconstructions with Prepectoral Implant. Plast Reconstr Surg 2016;137:1702-5. [Crossref] [PubMed]
- Kobraei EM, Cauley R, Gadd M, et al. Avoiding Breast Animation Deformity with Pectoralis-Sparing Subcutaneous Direct-to-Implant Breast Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e708. [Crossref] [PubMed]
- Gfrerer L, Liao EC. Technique Refinement in Prepectoral Implant Breast Reconstruction with Vicryl Mesh Pocket and Acellular Dermal Matrix Support. Plast Reconstr Surg Glob Open 2018;6:e1749. [Crossref] [PubMed]
- Nahabedian MY. Current Approaches to Prepectoral Breast Reconstruction. Plast Reconstr Surg 2018;142:871-80. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Sbitany H, Piper M, Lentz R. Prepectoral Breast Reconstruction: A Safe Alternative to Submuscular Prosthetic Reconstruction following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;140:432-43. [Crossref] [PubMed]
- Glasberg SB. The Economics of Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:49S-52S. [Crossref] [PubMed]
- Wirth GA, Mowlds DS, Guidotti P, et al. Acellular dermal matrix fenestrations and their effect on breast shape. Eur J Plast Surg 2015;38:267-72. [Crossref]
- Paydar KZ, Wirth GA, Mowlds DS. Prepectoral Breast Reconstruction with Fenestrated Acellular Dermal Matrix: A Novel Design. Plast Reconstr Surg Glob Open 2018;6:e1712. [Crossref] [PubMed]


