
The effect of intrathyroidal versus intraperitoneal bevacizumab on thyroid volume and vasculature flow in a rat model
Introduction
Thyroidectomy is one of the most common operations performed in the United States (1). Intraoperative blood loss is a concerning factor for head and neck endocrine surgeons performing thyroidectomy, especially in the setting of increased vascularity. Lugol’s solution has been utilized as a preoperative intervention to minimize any increase in thyroid vascularity prior to thyroidectomy. In a prospective study of 36 Graves patients, decreased blood flow, blood loss and microvessel density were observed (2). Based on the observation that thyroid perfusion decreased in those undergoing treatment with bevacizumab for other cancers (3), we apply this knowledge to the preoperative thyroidectomy workup.
We sought to identify a potential alternative treatment option to Lugol’s solution, bevacizumab. The overall objective was to determine if the administration of bevacizumab, known to reduce angiogenesis in other clinical settings, has any effect on thyroid volume, superior thyroid artery (STA) flow velocity, or vascular endothelial growth factor expression (VEGF) at thyroidectomy in the normal rat thyroid gland. The secondary aim is to identify if there is a difference between systemic versus local administration of bevacizumab.
The mouse model has been utilized to study the effects of bevacizumab on several thyroid cancers (4-6). Further, the rat model has been used to study thyroid pathophysiology as well (7-9). Due to the larger size, rats were chosen in order to make thyroidectomy easier.
Methods
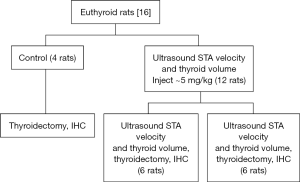
A total of 16 female euthyroid adult Sprague-Dawley rats were divided into three groups: 4 control rats, 6 rats administered bevacizumab intraperitoneal (IP) and 6 rats administered bevacizumab intrathyroidal (IT) via ultrasound guidance (Figure 1). Female rats were chosen due to the higher prevalence of thyroid conditions in the human female population. The rats were 200–300 grams in weight, large enough that central neck surgery is feasible. The University of Tennessee Institutional Animal Care and Use Committee approved this study.
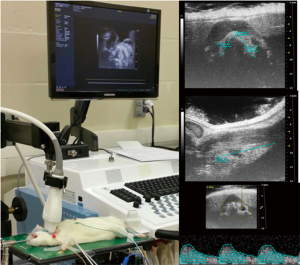
After a 3-day acclimation period each rat was placed under general anesthesia with spontaneous respirations. After inhalation of 5% isoflurane in a vented chamber for induction, IP ketamine (100 µg/g) and xylazine (0.5 µg/g) were administered to induce anesthesia. A nose cone allowing repeated exposure to inhalational isoflurane was fitted to prevent premature emergence from anesthesia. A small amount of electrode gel was placed on the animal’s four feet and they were secured to the platform with tape. A rectal temperature probe was inserted after lubricating with gel. Throughout the procedure, anesthetic depth, heart rate, body temperature, ECG, and respiratory rate were monitored using the Visual Sonics Vevo 2100 system. The animal maintained a body temperature of 37.5±0.5 °C, heart rate 450±50 bpm, and a normal QRST wave throughout the procedure. Each experimental rat was placed supine on the operating surface fitted for use with the Vevo 2100 high-frequency ultrasound imaging equipment, which is also fitted to record electrocardiography/monitor heart rate. The anterior neck hair was removed from the rat using depilatory cream. Ultrasound gel, which was centrifuged for clarity, was placed within the operative field and a 40 mHz linear array transducer positioned in contact with the gel (Figure 2). The STA flow velocity as well as the thyroid gland dimensions were obtained. The total thyroid volume was then calculated for each lobe and added together via the ellipsoid equation:
V (ml) = pi/6 × d × w × l
The experimental rats were injected after their initial ultrasound measurements, 6 local, IT injections and 6 systemic, IP injections. Each of the experimental animals was administered 1.25 mg bevacizumab and corresponds with a dose of 4–5 mg/kg. Two weeks were allowed to pass and the ultrasound was repeated with the above measurements obtained and compared. Two weeks was chosen as this timeframe correlates with both the administration time of Lugol’s solution (10–14 days) and other similar studies that have utilized bevacizumab. At this second terminal anesthesia encounter, thyroidectomy and thoracotomy were performed. The animals were then monitored for loss of heartbeat and confirmed death. The four control rats did not undergo ultrasound measurement, only the terminal procedure with pathologic analysis in order to serve as controls.
Thyroids were placed in fixative, processed and paraffin embedded, and sectioned into 8 µm-thick sections. One set of sections were mounted on slides, stained with hematoxylin and eosin (H&E), and imaged using light microscopy (Leica DMRXA2, Leica Microsystems, Bannockburn, IL, USA) and image capture software (SimplePCI, Hamamatsu Corp., Shizuoka, Japan). For fluorescence immunocytochemistry, another set of sections were treated after deparaffinization using heat-mediated antigen retrieval by boiling in 10 mM sodium citrate, pH 6.0 with 0.05% Tween-20 for 1 hour. After cooling, slides were washed in PBS and subsequently blocked in 10% goat serum/5% BSA/0.5% TritonX-100 in PBS for 1 hour at RT. Following blocking, sections were incubated with rabbit anti-CD31/PECAM (Novus Biologicals, NB100-2284) and mouse anti-VEGF (Santa Crus Biotechnologies, sc-7269) in the same buffer overnight at 4 °C. After washing in PBS, slides were probed using goat anti-rabbit IgG conjugated to AlexaFluor 488 (Invitrogen, A-11034), goat anti-mouse IgG conjugated to AlexaFluor 555 (Invitrogen, 21422), and DAPI (Invitrogen, D-1306). After washing in PBS, slides were mounted using Prolong Diamond Anti-Fade Mountant (Invitrogen, P-36961). Control sections with the primary antibodies omitted had no visible staining. Some images were captured as Z-stacks and imaged using a Zeiss 710 Laser Scanning Confocal Microscope; other images were obtained using the Leica episcopic-fluorescent microscope mentioned above. All images were obtained using the same laser power (or camera exposure time) and gain settings to ensure comparability.
To analyze possible group differences in VEGF and CD31, software (ImageJ 1.48v, National Institutes of Health, USA) was used to split the images into their component color channels (red, green and blue), and measure the average brightness within 300×300 ppi ROIs, which were chosen as areas of maximum signal within an image. These measurements were expressed as a ratio with respect to brightness of the DAPI signal {which did not differ significantly among groups; one-way ANOVA, F[2,15] =0.004, P=0.99} to control for possible overall differences in section brightness. Data were analyzed with one-way ANOVA.
Results
Ultrasound evaluation
The experimental groups did not differ in body weight (267 vs. 268 g, P=0.81), thyroid volume (62 vs. 55, P=0.34), peak STA velocity (76 vs. 73 mm/s, P=0.87), or average STA velocity (46 vs. 45 mm/s, P=0.95).
When comparing the pre and post IP bevacizumab groups (Table 1), no difference in body weight (266.7 vs. 269.8, P=0.64) was found. Both right (25.455 vs. 18.983, P=0.05) and left (36.128 vs. 27.504, P=0.04) thyroid lobe volumes were decreased post IP bevacizumab corresponding to a total thyroid volume decrease (62.583 vs. 42.161, P=0.004). No significant differences occurred with blood flow measurements for peak velocity (75.896 vs. 76.7, P=0.96), average velocity (45.748 vs. 43.867, P=0.88), or RI (30.345 vs. 25.32, P=0.60).
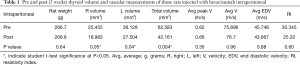
Full table
In the comparison between the pre and post IT bevacizumab (Table 2), no difference in weight (268.3 vs. 270.7 g, P=0.74) nor thyroid volume (55.229 vs. 58.16, P=0.64) was found. The average peak (73.191 vs. 100.589 cm/s, P=0.03) and mean (45.047 vs. 62.843 m/s, P=0.03) velocities were increased in the post IT bevacizumab group, but did not differ in the RI (0.619 vs. 0.632, P=0.82).
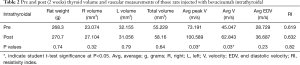
Full table
Histology
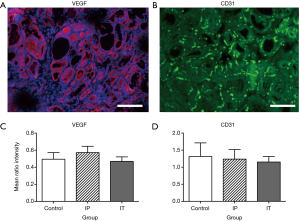
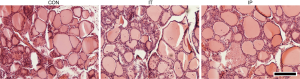
Examples of immunofluorescence for VEGF and CD31, and quantification of signal brightness for each experimental group, are shown in Figure 3. There were no significant differences among groups in either VEGF expression {F[2,15] =0.69, P<0.52} or for CD31 expression {F[2,15] =0.083, P<0.92}. Likewise, there was no apparent qualitative difference in overall microscopic thyroid structure as revealed by examination of H&E staining (Figure 4).
Discussion
Bevacizumab was first described in 1993 (in the journal Nature). Since then, many applications of this drug have been demonstrated (e.g., in treating colon cancer, lung cancer, and macular degeneration). The use of this medication has been increasing in prevalence and is now used in the otolaryngology realm for treatment of recurrent respiratory papillomatosis.
In our study, we found via ultrasound examination that thyroid volume decreased in the IP administration group. This deserves further investigation and suggests that bevacizumab has an effect on thyroid physiology. We also noted an increase in STA flow velocity in the IT group; however, the resistivity index did not increase which attenuates the significance of this result. It is possible that one administration of the drug may not be enough to obtain such an effect. Additionally, no apparent changes in microscopic vasculature of the thyroid as revealed by VEGF or CD31 immunohistology were noted after a single administration of bevacizumab. Again, robust changes may only be evident with repeated dosing of the drug.
Although our study obtained limited results, we believe further dose titration studies should be investigated for the use of bevacizumab in prior thyroidectomy situations such as thyroid goiter and Graves disease.
Conclusions
Single systemic administration of bevacizumab appears to decrease thyroid volume. Single IT injection may increase thyroid blood flow. These preliminary findings support further study of pharmacologic intervention with bevacizumab in thyroid conditions characterized by increased angiogenesis and vascularity such as Graves disease and iodine deficiency. Dosing administration and titration should be explored.
Acknowledgments
We would like to thank the UTHSC Samuel Sanders Otolaryngology Resident Research Fund for funding this investigation.
Footnote
Conflicts of Interest: Other Presentations—This manuscript was given as a poster presentation at that American Academy of Otolaryngology Head and Neck Surgery Annual Meeting in Chicago, IL, USA.
Ethical Statement: The study was approved by The University of Tennessee Institutional Animal Care and Use Committee.
References
- Biron VL, Bang H, Farwell DG, et al. National Trends and Factors Associated with Hospital Costs Following Thyroid Surgery. Thyroid 2015;25:823-9. [Crossref] [PubMed]
- Erbil Y, Ozluk Y, Giriş M, et al. Effect of lugol solution on thyroid gland blood flow and microvessel density in the patients with Graves' disease. J Clin Endocrinol Metab 2007;92:2182-9. [Crossref] [PubMed]
- van der Veldt AA, Lammertsma AA, Smit EF. Reduction in thyroid perfusion after bevacizumab treatment. Thyroid 2013;23:1329-30. [Crossref] [PubMed]
- Prichard CN, Kim S, Yazici YD, et al. Concurrent cetuximab and bevacizumab therapy in a murine orthotopic model of anaplastic thyroid carcinoma. Laryngoscope 2007;117:674-9. [Crossref] [PubMed]
- Salaun PY, Bodet-Milin C, Frampas E, et al. Toxicity and efficacy of combined radioimmunotherapy and bevacizumab in a mouse model of medullary thyroid carcinoma. Cancer 2010;116:1053-8. [Crossref] [PubMed]
- Yang Y, Zhang Y, Cao Z, et al. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc Natl Acad Sci U S A 2013;110:12018-23. [Crossref] [PubMed]
- Fernandez Rodriguez A, Galera Davidson H, Salguero Villadiego M, et al. Induction of thyroid proliferative changes in rats treated with antithyroid compound. Anat Histol Embryol 1991;20:289-98. [Crossref] [PubMed]
- Kong L, Wei Q, Fedail JS, et al. Effects of thyroid hormones on the antioxidative status in the uterus of young adult rats. J Reprod Dev 2015;61:219-27. [Crossref] [PubMed]
- McAllister RM, Albarracin I, Price EM, et al. Thyroid status and nitric oxide in rat arterial vessels. J Endocrinol 2005;185:111-9. [Crossref] [PubMed]


