Core needle biopsy in the management of thyroid nodules with an indeterminate fine-needle aspiration report
Introduction
Ultrasonography (US)-guided fine-needle aspiration biopsy (FNAB) cytology is widely used as a minimally-invasive tool for evaluating thyroid lesions (1,2). However, FNAB is limited due to pathologically indeterminate results in about 10 to 20% of the cases (3). Even though the American Thyroid Association (ATA) guidelines recommend repeat FNAB for these cases, repeat FNAB shows 1–7% of non-diagnostic results and 3.8–31.0% of indeterminate results (4-7). It is known that the malignancy risk of the AUS/FLUS category is 6–18% based on the 2017 Bethesda system (7). However, there has been a tendency to overuse the diagnosis of AUS/FLUS and the reported malignancy risk varies from the proposed rate by 14% to 38% (8,9).
Due to the variable risk of malignancy of these indeterminate thyroid nodules, other guidelines (2,10) recommend evaluating these nodules by diagnostic surgery. However, the majority (70–80%) of indeterminate thyroid nodules are benign according to surgical histology (11). Other solutions for dealing with indeterminate thyroid nodules, such as the molecular testing or gene expression classifier, or clinical studies together with US findings are investigated, but such parameters are still controversial (12-15). Recently, core-needle biopsy (CNB) was introduced as a safe and effective tool in diagnosing indeterminate thyroid nodules in order to prevent unnecessary surgery (16,17). Some studies reported that as much as 98% of indeterminate thyroid nodules are able to be classified as malignant or benign when CNB is used for follow-up analysis (18-22).
Therefore, in this review, we investigate how to deal with indeterminate thyroid nodules diagnosed by FNAB and using CNB.
Strategies to deal with indeterminate FNAB results
Various additional tests have been suggested for thyroid nodules with previously indeterminate FNAB results, such as repeat FNAB (23), molecular testing (12-15), diagnostic surgery (2,10) or CNB (16,17,24). The recently revised ATA management guidelines proposed that repeat FNAB or molecular testing be used and in case the results are inconclusive, that excision be performed with consideration of the clinical and radiologic features and patient preferences (25).
Repeated FNAB can be easily performed if the primary FNAB result is indeterminate, however, the benefit in patient management is still unclear because there is still a chance to obtain indeterminate results (i.e., non-diagnostic and AUS/FLUS results) (26). Only a small chance to diagnose malignancy (1.35% to 24.77%) is reported (6,9,27-32). Application of immunohistochemical stains such as galectin-3, cytokeratin-19, HBME-1, or BRAFV600E has been introduced for these nodules (33,34). The most important drawback is that immunohistochemical stains do not guarantee significant improvement in the diagnostic accuracy. Diagnostic surgery can provide a definitive diagnosis, although the procedure itself not only has a risk of complications but also holds a high possibility for benign results (11).
Some recent studies showed that histologic examinations by CNB can accurately diagnose a large percentage of indeterminate thyroid nodules (16,17) and with a very low rate of minor complications (35). Although CNB has a risk of repeated indeterminate results, CNB demonstrated a summary sensitivity of 91% (95% CI, 81–96%) and specificity of 99% (95% CI, 98–100%) by a meta-analysis using data collected from 10 CNB studies with 1,733 patients (36).
Recently, a web-based risk stratification system created using combination of Bethesda III thyroid nodules and US features has been introduced (http://www.gap.pe.kr/xe/Estimation_A) (37). This system is based on a previously published web-based prediction model (http://www.gap.pe.kr/xe/Estimation) (38), which is a simple and easily accessible web-based diagnostic scoring system for the malignancy risk stratification of thyroid nodules. Evaluation of the malignancy risk-stratification system showed good predictive accuracy, with approximate AUCs of 0.83.
CNB in diagnosing indeterminate thyroid nodules
Although it is recorded in the literature that CNB was performed for thyroid nodules in the early 1980s, large-needle biopsy, conducted without US guidance was not recognized in clinical use due to the pain and bleeding risk at that time (39,40). With the widespread use of high-resolution US and introduction of advanced CNB devices such as spring-activated single- or double-action core needles, accurate diagnosis became possible using US-guided CNB with a minimal chance of complications (41). Since then CNB has been reported as an effective and safe method in diagnosing thyroid nodules (18,26,42-44).
By obtaining enough tissue of the nodules using CNB, more information regarding the architectural histologic structure such as the nodule capsule or more immunochemical staining can be obtained (45). In addition, CNB has the advantage of assessing nuclear change, general alterations in the follicular structure, and relationships with adjacent tissues by obtaining adequate material from the nodule (43). We can expect the potential of overcoming the limitation of FNAB, such as poor specimen quality of cellularity and appropriate preservation which leads to misdiagnoses (26,33,46-48). Although several studies revealed that CNB demonstrates no additional benefit to that of FNAB (41,49,50), the role of CNB has been suggested in many recent studies (18,26,46). Up to 98% of indeterminate nodules can be classified as malignant or benign when CNB is performed (18-22).
A recent article investigated that subcategory nodules of nuclear atypia had a higher malignancy risk, of being surgical candidates, of having suspicious US features, and of having malignant CNB results than subcategory nodules of architectural atypia (51). Other studies showed that CNB was helpful for diagnosing subcategory nodules of nuclear atypia but was not, or less helpful for subcategory nodules of architectural atypia (49,52,53). Yet at the same time, another study of 153 consecutive patients suggested that CNB might be more useful in decision making than repeat FNAB in both subcategory nodules of nuclear atypia and subcategory nodules of architectural atypia and it has the potential to be a first-line alternative diagnostic tool for initially diagnosed indeterminate thyroid nodules (53).
Even though there is no clear guideline for CNB in diagnosing indeterminate thyroid nodules yet, evidences revealed the effectiveness of CNB for reducing inconclusive results and improving the diagnostic performance of thyroid nodules with initial indeterminate results (54). In indeterminate lesions, the combined use of repeated FNAB and CNB might be considered. Previous studies with large series of nodules showed that CNB has higher accuracy than repeated FNAB, although the combination of two biopsies even improves the rate of diagnosis (26,46).
Several studies have shown the usefulness of CNB for thyroid nodules with AUS/FLUS results. In a retrospective study comparing three management tools i.e., CNB, repeat FNAB, and diagnostic surgery for previous AUS/FLUS in FNAB, the CNB results were preferable, i.e., 77.8% benign, 20.3% cancer, and 1.8% non-diagnostic, than those of repeat FNAB i.e., 35.2% benign, 16.1% cancer, and 48.6% non-diagnostic, and were comparable to those of diagnostic surgery (18). In a prospective study of concurrent CNB and FNAB, the incidence of non-diagnostic or AUS/FLUS results was lower in CNB, i.e., 3.1% non-diagnostic and 23.6% AUS/FLUS results than in repeat FNAB, i.e., 9.3% non-diagnostic and 39.8% AUS/FLUS results (26).
A meta-analysis by Suh et al. (36) demonstrated that CNB showed higher sensitivity (91%) in diagnosing malignancy than FNA (74%) and with no significant difference in specificity i.e., 99% vs. 100% respectively, and a lower pooled proportion of non-diagnostic results compared with FNAB (5.5% vs. 22.6%). CNB showed much fewer inconclusive results than FNAB and with a pooled proportion of 8.0% (95% CI, 4.4–11.5%) vs. a pooled proportion of 40.2% (95% CI, 25.1–55.3%). Therefore, the authors argue that CNB may be a complementary diagnostic tool in nodules with initially non-diagnostic and AUS/FLUS results on previous FNAB. Also, the National Cancer Institute (40), American Association of Clinical Endocrinologists/American College of Endocrinology/Associazione Medici Endocrinologi (AACE/ACE/AME) (1), and the Korean Society of Thyroid Radiology (KSThR) (55) proposed CNB as an additional diagnostic strategy for thyroid nodules with previous non-diagnostic FNAB results.
CNB guidelines for Bethesda III and IV according to 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology (KSThR)
CNB for atypia (follicular lesion) of undetermined significance in previous FNAB
Even though the malignancy risk of the AUS/FLUS category varies between 15–25% based on the Bethesda system (10), there has been a tendency to overuse the diagnosis of indeterminate and the reported malignancy risk varies from the proposed rate, to reach 14% to 38% (8,9). To improve the accuracy in detection of malignancy and to make better management decision for the AUS/FLUS category, various trials have been suggested such as application of immunohistochemical stains or repeat FNAB or CNB or even diagnostic surgery (33,34). A study reported that CNB results were better in diagnosing thyroid nodules than those of repeat FNAB and equivalent with those of surgical excision (18). Also, a prospective study revealed that the incidence of non-diagnostic or AUS/FLUS was lower in CNB than in repeat FNAB (26). Thus, based on these references, recommendation of the KSThR is to use CNB as good alternative to FNAB for indeterminate thyroid nodules.
CNB for follicular neoplasms
Since follicular neoplasm is challenging to diagnose with FNAB, CNB has been introduced as a complementary method for thyroid nodules because the enough specimen obtained can make detailed histologic evaluation and ancillary immunohistochemical staining possible (26,47,56). Also, ever since new sampling techniques including the capsule of the nodule and the surrounding extranodular parenchyma as well as nodular tissue were introduced, follicular neoplasm and unencapsulated non-neoplastic nodules could be distinguished by identifying the presence of a fibrous capsule on histologic evaluation (57,58). Thus, KSThR recommended CNB for follicular neoplasm as follows (55): (I) CNB can differentiate non-neoplastic nodules from encapsulated follicular neoplasms, (II) CNB cannot differentiate follicular thyroid carcinoma from follicular adenoma.
Limitations of CNB
Although CNB shows high efficacy and safety with competent accuracy, one study demonstrated that up to 36% of the results remained indeterminate after CNB due to insufficient cytology to differentiate nodular hyperplasia from follicular neoplasm (52). Another concern regarding CNB is the possibility of false-negative result. As the tissue is only collected by the side hole of the needle, there is a possibility of capturing the normal thyroid tissue, not the target lesion (18).
Furthermore, no clear guidelines regarding the management of AUS/FLUS results are also limitation of CNB. AACE/ACE/AME guidelines do not recommend the use of CNB in indeterminate nodules due to the limited evidence and the lack of validated reporting systems (59). However, the recently suggested pathology reporting system by the Korean endocrine pathology thyroid CNB study group is expected to be useful in diagnosing CNB specimens (60,61).
Safety of CNB
A retrospective study evaluated 6,169 consecutive patients with 6,687 thyroid nodules and showed overall 53 complications in 50 patients (0.81%), including four major complications including massive hematoma, pseudoaneurysm or voice problems (62).
According to a systematic review and meta-analysis evaluated complications following CNB (36), there was only one major complication among 3,163 patients (0.03%), and which was overnight hospitalization for observation of bleeding. There were 15 minor complications after CNB among 2,608 patients (0.58%), including 12 patients with hematomas, two patients with transient hoarseness, and one patient with hemoptysis. On the other hand, there were no major complications after FNAB in 2,572 patients and the three, minor complications in 2,017 patients include two with hematomas and one patient with transient hoarseness. Other studies controlled post-CNB perinodular hemorrhage with simple manual compression (58,63). Pain during biopsies was controllable with local anesthesia (63).
Several studies compared the complications of FNAB and CNB. A recent study showed no significant differences when comparing the two procedures in terms of pain, tolerability, or complications (64). Two previous studies have also shown similar results regarding the tolerability and pain between the two procedures (35,65).
Cost-effectiveness of CNB
A recent study demonstrated the cost-effectiveness of CNB regarding avoiding unnecessary diagnostic surgery for thyroid nodules with indeterminate pathologic results on FNAB (66). In this study, the authors classified 42.4% of the indeterminate FNAB resulting thyroid nodules as benign according to CNB. This study demonstrated that the cost of a single CNB is less expensive than diagnostic surgery at about 1/6. They also insisted that about one-third of the expense can be saved with CNB compared to undergoing diagnostic thyroidectomy for all nodules with indeterminate results on FNAB by avoiding unnecessary diagnostic surgery.
CNB technical perspective
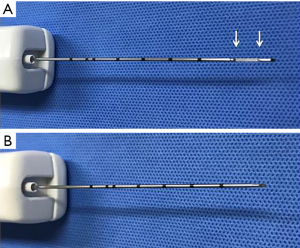
CNB needs to be performed by experienced operators with US guidance. Operators should decide the suitable CNB needles and the approach route via pre-procedural US to improve the safety and diagnostic accuracy (55). The trans-isthmic approach is usually considered suitable, but operators should completely understand and plan the most appropriate access route. It is also recommended to choose a needle with a similar length with the targeted nodules (26,46) (Figure 1). For the optimal result, the entire needle should be scanned during the procedure and the needles have to be remained parallel to the US probe axis during the procedure. Before firing the core needle, operators should envision the anticipated needle route and make sure that the needle tip would be in the safe place. The location of the specimen notch is adjustable after firing the stylet select most proper sampling target.
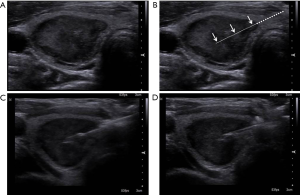
There are two strategies for obtaining a specimen with CNB. One is to locate the specimen notch of the biopsy needle confined to the internal portion of the thyroid nodule (58). The other is to biopsy the capsule of the thyroid nodule and the surrounding parenchyma (57) in order to adequately diagnose follicular-pattern lesions (63). Using this modified CNB technique, three components should be obtained for the pathological interpretation, i.e., nodule tissue, nodule-parenchyma border including capsule, and normal thyroid parenchyma (57,67) so as to differentiate nodular hyperplasia from follicular neoplasm (58) (Figure 2). For sampling hard nodules such as severe fibrosis or dense calcification, it is necessary to stab the nodule in order to first identify a weak point. To prevent surrounding structure damage due to needle deflection during the hard nodule CNB, careful evaluation of surrounding structures is vital (68-70).
Visual assessment should be performed in order to confirm whether additional CNB is required (26,46,69,71) after the first CNB. After the visual inspection, the tissue has to be immediately fixed in formalin. In most cases, one or two biopsy sampling is sufficient for the adequate histology diagnosis. A study suggested to obtain at least two core specimens including an intranodular and capsule portion when using a 1.1-cm core device (72), while other researchers argued that one specimen would be enough when using a longer core device, i.e., 1.6- or 2-cm (73). When a nodule has heterogeneous components in US, it is better to harvest tissue in multiple sites of the nodule in order to represent all areas of the nodule. Just in case complication such as bleeding happens, additional sampling could be postponed. Manual compression should be carried out for 20 to 30 minutes immediately after the biopsy.
Conclusions
CNB is safe and effective in diagnosing thyroid nodules. The wide use of US-guided CNB for previous indeterminate FNAB results may be the next successful diagnostic tool in order to reduce unnecessary surgeries.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract 2010;16:468-75. [Crossref] [PubMed]
- Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014;81:1-122. [Crossref] [PubMed]
- Baloch ZW, Fleisher S. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn cytopathol 2002;26:41-4. [Crossref] [PubMed]
- Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration. Cancer 2009;117:195-202. [PubMed]
- Yang J, Schnadig V, Logrono R, et al. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer 2007;111:306-15. [Crossref] [PubMed]
- Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer 2007;111:508-16. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid 2017;27:1341-6. [Crossref] [PubMed]
- Heller KS. Malignancy rate in thyroid nodules classified as Bethesda category III (AUS/FLUS): is there a correct answer? Thyroid 2014;24:787-8. [Crossref] [PubMed]
- Ho AS, Sarti EE, Jain KS, et al. Malignancy rate in thyroid nodules classified as Bethesda category III (AUS/FLUS). Thyroid 2014;24:832-9. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol 2009;132:658-65. [Crossref] [PubMed]
- Trimboli P, Treglia G, Guidobaldi L, et al. Clinical characteristics as predictors of malignancy in patients with indeterminate thyroid cytology: a meta-analysis. Endocrine 2014;46:52-9. [Crossref] [PubMed]
- Trimboli P, Virili C, Romanelli F, et al. Galectin-3 performance in histologic a cytologic assessment of thyroid nodules: a systematic review and meta-analysis. Int J Mol Sci 2017;18:1756. [Crossref] [PubMed]
- Trimboli P, Condorelli E, Catania A, et al. Clinical and ultrasound parameters in the approach to thyroid nodules cytologically classified as indeterminate neoplasm. Diagn Cytopathol 2009;37:783-5. [Crossref] [PubMed]
- Trimboli P, Fulciniti F, Zilioli V, et al. Accuracy of international ultrasound risk stratification systems in thyroid lesions cytologically classified as indeterminate. Diagn Cytopathol 2017;45:113-7. [Crossref] [PubMed]
- Saggiorato E, Angusti T, Rosas R, et al. 99mTc-MIBI Imaging in the presurgical characterization of thyroid follicular neoplasms: relationship to multidrug resistance protein expression. J Nucl Med 2009;50:1785-93. [Crossref] [PubMed]
- Trimboli P, Crescenzi A. Thyroid core needle biopsy: taking stock of the situation. Endocrine 2015;48:779-85. [Crossref] [PubMed]
- Trimboli P, Giovanella L. Reliability of core needle biopsy as a second-line procedure in thyroid nodules with an indeterminate fine-needle aspiration report: a systematic review and meta-analysis. Ultrasonography 2018;37:121-8. [Crossref] [PubMed]
- Park KT, Ahn SH, Mo JH, et al. Role of core needle biopsy and ultrasonographic finding in management of indeterminate thyroid nodules. Head Neck 2011;33:160-5. [Crossref] [PubMed]
- Bartolazzi A, Orlandi F, Saggiorato E, et al. Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: a prospective multicentre study. Lancet Oncol 2008;9:543-9. [Crossref] [PubMed]
- Saggiorato E, De Pompa R, Volante M, et al. Characterization of thyroid ‘follicular neoplasms’ in fine-needle aspiration cytological specimens using a panel of immunohistochemical markers: a proposal for clinical application. Endocr Relat Cancer 2005;12:305-17. [Crossref] [PubMed]
- Treglia G, Caldarella C, Saggiorato E, et al. Diagnostic performance of 99m Tc-MIBI scan in predicting the malignancy of thyroid nodules: a meta-analysis. Endocrine 2013;44:70-8. [Crossref] [PubMed]
- Trimboli P, Treglia G, Sadeghi R, et al. Reliability of real-time elastography to diagnose thyroid nodules previously read at FNAC as indeterminate: a meta-analysis. Endocrine 2015;50:335-43. [Crossref] [PubMed]
- Jooya A, Saliba J, Blackburn A, et al. The role of repeat fine needle aspiration in the management of indeterminate thyroid nodules. J Otolaryngol Head Neck Surg 2016;45:51. [Crossref] [PubMed]
- Baek JH. Current status of core needle biopsy of the thyroid. Ultrasonography 2017;36:83. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Na DG, Kim J-h, Sung JY, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid 2012;22:468-75. [Crossref] [PubMed]
- Wong LQ. LiVolsi VA, Baloch ZW. Diagnosis of atypia/follicular lesion of undetermined significance: An institutional experience. Cytojournal 2014;11:23. [Crossref] [PubMed]
- Dincer N, Balci S, Yazgan A, et al. Follow-up of atypia and follicular lesions of undetermined significance in thyroid fine needle aspiration cytology. Cytopathology 2013;24:385-90. [Crossref] [PubMed]
- Baloch Z. Role of repeat fine-needle aspiration biopsy (FNAB) in the management of thyroid nodules. Diagn Cytopathol 2003;29:203-6. [Crossref] [PubMed]
- Faquin WC, Baloch ZW. Fine-needle aspiration of follicular patterned lesions of the thyroid: diagnosis, management, and follow-up according to National Cancer Institute (NCI) recommendations. Diagn Cytopathol 2010;38:731-9. [PubMed]
- Graciano AJ, Chone CT, Fischer CA, et al. Repeated fine-needle aspiration cytology for the diagnosis and follow-up of thyroid nodules. Braz J Otorhinolaryngol 2014;80:422-7. [Crossref] [PubMed]
- Vanderlaan PA, Krane JF, Cibas ES. The frequency of 'atypia of undetermined significance' interpretations for thyroid fine-needle aspirations is negatively correlated with histologically proven malignant outcomes. Acta Cytol 2011;55:512-7. [Crossref] [PubMed]
- Trimboli P, Guidobaldi L, Amendola S, et al. Galectin-3 and HBME-1 improve the accuracy of core biopsy in indeterminate thyroid nodules. Endocrine 2016;52:39-45. [Crossref] [PubMed]
- Choi SH, Baek JH, Lee JH, et al. Evaluation of the clinical usefulness of BRAFV600E mutation analysis of core-needle biopsy specimens in thyroid nodules with previous atypia of undetermined significance or follicular lesions of undetermined significance results. Thyroid 2015;25:897-903. [Crossref] [PubMed]
- Nasrollah N, Trimboli P, Rossi F, et al. Patient’s comfort with and tolerability of thyroid core needle biopsy. Endocrine 2014;45:79-83. [Crossref] [PubMed]
- Suh CH, Baek JH, Lee JH, et al. The role of core-needle biopsy in the diagnosis of thyroid malignancy in 4580 patients with 4746 thyroid nodules: a systematic review and meta-analysis. Endocrine 2016;54:315-28. [Crossref] [PubMed]
- Choi YJ, Baek JH, Shin JH, et al. Web-based thyroid imaging reporting and data system: Malignancy risk of atypia of undetermined significance or follicular lesion of undetermined significance thyroid nodules calculated by a combination of ultrasonography features and biopsy results. Head Neck 2018;40:1917-25. [Crossref] [PubMed]
- Choi YJ, Baek JH, Baek SH, et al. Web-based malignancy risk estimation for thyroid nodules using ultrasonography characteristics: development and validation of a predictive model. Thyroid 2015;25:1306-12. [Crossref] [PubMed]
- Moon W-J, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation—multicenter retrospective study. Radiology 2008;247:762-70. [Crossref] [PubMed]
- Baloch ZW, Cibas ES, Clark DP, et al. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: a summation. Cytojournal 2008;5:6. [Crossref] [PubMed]
- Novoa E, Gürtler N, Arnoux A, et al. Role of ultrasound-guided core-needle biopsy in the assessment of head and neck lesions: a meta-analysis and systematic review of the literature. Head Neck 2012;34:1497-503. [Crossref] [PubMed]
- Liu Q, Castelli M, Gattuso P, et al. Simultaneous fine-needle aspiration and core-needle biopsy of thyroid nodules. Am Surg 1995;61:628-32; discussion 632-3. [PubMed]
- Renshaw AA, Pinnar N. Comparison of thyroid fine-needle aspiration and core needle biopsy. Am J Clin Pathol 2007;128:370-4. [Crossref] [PubMed]
- Screaton NJ, Berman LH, Grant JW. US-guided core-needle biopsy of the thyroid gland. Radiology 2003;226:827-32. [Crossref] [PubMed]
- Kim JH, Na DG, Lee H. Ultrasonographic Echogenicity and Histopathologic Correlation of Thyroid Nodules in Core Needle Biopsy Specimens. Korean J Radiol 2018;19:673-81. [Crossref] [PubMed]
- Sung JY, Na DG, Kim KS, et al. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol 2012;22:1564-72. [Crossref] [PubMed]
- Crescenzi A, Trimboli P, Modica DC, et al. Preoperative assessment of TERT promoter mutation on thyroid core needle biopsies supports diagnosis of malignancy and addresses surgical strategy. Horm Metab Res 2016;48:157-62. [PubMed]
- Raab SS, Vrbin CM, Grzybicki DM, et al. Errors in thyroid gland fine-needle aspiration. Am J Clin Pathol 2006;125:873-82. [Crossref] [PubMed]
- Hakala T, Kholová I, Sand J, et al. A core needle biopsy provides more malignancy-specific results than fine-needle aspiration biopsy in thyroid nodules suspicious for malignancy. J Clin Pathol 2013;66:1046-50. [Crossref] [PubMed]
- Khoo T-K, Baker C, Hallanger-Johnson J, et al. Comparison of ultrasound-guided fine-needle aspiration biopsy with core-needle biopsy in the evaluation of thyroid nodules. Endocr Pract 2008;14:426-31. [Crossref] [PubMed]
- Choi YJ, Baek JH, Ha EJ, et al. Differences in risk of malignancy and management recommendations in subcategories of thyroid nodules with atypia of undetermined significance or follicular lesion of undetermined significance: the role of ultrasound-guided core-needle biopsy. Thyroid 2014;24:494-501. [Crossref] [PubMed]
- Hahn SY, Shin JH, Han BK, et al. Ultrasonography-guided core needle biopsy for the thyroid nodule: does the procedure hold any benefit for the diagnosis when fine-needle aspiration cytology analysis shows inconclusive results? Br J Radiol 2013;86:20130007. [Crossref] [PubMed]
- Na DG, Min HS, Lee H, et al. Role of core needle biopsy in the management of atypia/follicular lesion of undetermined significance thyroid nodules: comparison with repeat fine-needle aspiration in subcategory nodules. Eur Thyroid J 2015;4:189-96. [Crossref] [PubMed]
- Choi YJ, Baek JH, Suh CH, et al. Core-needle biopsy versus repeat fine-needle aspiration for thyroid nodules initially read as atypia/follicular lesion of undetermined significance. Head Neck 2017;39:361-9. [Crossref] [PubMed]
- Na DG, Baek JH, Jung SL, et al. Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean J Radiol 2017;18:217-37. [Crossref] [PubMed]
- Crescenzi A, Guidobaldi L, Nasrollah N, et al. Immunohistochemistry for BRAF (V600E) antibody VE1 performed in core needle biopsy samples identifies mutated papillary thyroid cancers. Horm Metab Res 2014;46:370-4. [Crossref] [PubMed]
- Nasrollah N, Trimboli P, Guidobaldi L, et al. Thin core biopsy should help to discriminate thyroid nodules cytologically classified as indeterminate. A new sampling technique. Endocrine 2013;43:659-65. [Crossref] [PubMed]
- Han S, Shin J, Hahn S, et al. Modified core biopsy technique to increase diagnostic yields for well-circumscribed indeterminate thyroid nodules: a retrospective analysis. AJNR Am J Neuroradiol 2016;37:1155-9. [Crossref] [PubMed]
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules--2016 update. Endocr Pract 2016;22:622-39. [Crossref] [PubMed]
- Jung CK, Min HS, Park HJ, et al. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. J Pathol Transl Med 2015;49:288. [Crossref] [PubMed]
- Jung CK, Baek JH. Recent advances in core needle biopsy for thyroid nodules. Endocrinol Metab (Seoul) 2017;32:407-12. [Crossref] [PubMed]
- Ha EJ, Baek JH, Lee JH, et al. Complications following US-guided core-needle biopsy for thyroid lesions: a retrospective study of 6,169 consecutive patients with 6,687 thyroid nodules. Eur Radiol 2017;27:1186-94. [Crossref] [PubMed]
- Ahn S, Jung S, Kim J-Y, et al. Evaluation of Modified Core-Needle Biopsy in the Diagnosis of Thyroid Nodules. Korean J Radiol 2018;19:656-64. [Crossref] [PubMed]
- Jeong EJ, Chung SR, Baek JH, et al. A comparison of ultrasound-guided fine needle aspiration versus core needle biopsy for thyroid nodules: pain, tolerability, and complications. Endocrinol Metab (Seoul) 2018;33:114-20. [Crossref] [PubMed]
- Stangierski A, Wolinski K, Martin K, et al. Core needle biopsy of thyroid nodules–evaluation of diagnostic utility and pain experience. Neuro Endocrinol Lett 2013;34:798-801. [PubMed]
- Trimboli P, Nasrollah N, Amendola S, et al. A cost analysis of thyroid core needle biopsy vs. diagnostic surgery. Gland Surg 2015;4:307-11. [PubMed]
- López JI, Zabala R, del Cura JL. Histological diagnosis of thyroid disease using ultrasound-guided core biopsies. Eur Thyroid J 2013;2:29-36. [PubMed]
- Yi KS, Kim JH, Na DG, et al. Usefulness of core needle biopsy for thyroid nodules with macrocalcifications: comparison with fine-needle aspiration. Thyroid 2015;25:657-64. [Crossref] [PubMed]
- Ha EJ, Baek JH, Lee JH, et al. Core needle biopsy can minimise the non-diagnostic results and need for diagnostic surgery in patients with calcified thyroid nodules. Eur Radiol 2014;24:1403-9. [Crossref] [PubMed]
- Na DG, Kim DS, Kim SJ, et al. Thyroid nodules with isolated macrocalcification: malignancy risk and diagnostic efficacy of fine-needle aspiration and core needle biopsy. Ultrasonography 2016;35:212. [Crossref] [PubMed]
- Yeon JS, Baek JH, Lim HK, et al. Thyroid nodules with initially nondiagnostic cytologic results: the role of core-needle biopsy. Radiology 2013;268:274-80. [Crossref] [PubMed]
- Hahn SY, Shin JH, Oh YL. What is the ideal core number for ultrasonography-guided thyroid biopsy of cytologically inconclusive nodules? AJNR Am J Neuroradiol 2017;38:777-81. [Crossref] [PubMed]
- Park HS, Baek JH, Gyu ND. Regarding “What Is the Ideal Core Number for Ultrasonography-Guided Thyroid Biopsy of Cytologically Inconclusive Nodules?”. AJNR Am J Neuroradiol 2017;38:E53-4. [Crossref] [PubMed]