Long-term quality of voice is usually acceptable after initial hoarseness caused by a thyroidectomy or a parathyroidectomy
Introduction
Thyroid and parathyroid operations carry a small risk of injuring the recurrent laryngeal nerve (RLN) (1,2). It has traditionally been quoted by surgeons that the incidence of RLN injuries is 1–2% but this may be an underestimate and depends greatly upon the proportion of patients undergoing routine vocal cord (VC) assessment post-operatively, as stated by the latest British Association of Endocrine Surgeons (BAETS) Annual Report (3).
The time interval by which RLN palsy might be deemed persistent was traditionally considered to be 12 months while since the last revision of the BAETS database in October 2014, the outcome of any RLN palsy at 6 months post-operation has been the definition for persistence (3-5).
Unilateral injury of the RLN leads to glottis insufficiency and results in a number of symptoms including hoarseness, aspiration and swallowing difficulties (6). Bilateral injury to the RLN’s may cause potentially life threatening airway obstruction and in the vast majority of cases results in a tracheostomy to secure the airway. Depending on the nature of the RLN injury (temporary/permanent) and duration from the time of the injury, some of the symptoms may improve or resolve over time (5).
There is a significant paucity of high-quality data in the literature for post-operative voice changes and as a result, an absence of level I evidence coming from randomized controlled trials and/or meta-analysis of voice changes (5). The available evidence from single-centre studies are pointing to fact that postoperative voice impairment is more common that what it is widely believed; Page et al. in a study of 395 patients showed that 49% had voice impairment immediately after surgery and Grover et al. in 2013 showed that a third of patients had mild voice problems 1 year after surgery (7-10).
The goal of this study was to investigate the incidence of VC palsy in post-operative patients following an endocrine surgical operation (thyroidectomy/parathyroidectomy) in a tertiary referral institution, track the management of these cases and record the long-term outcomes and VC recovery rates.
Methods
This was a retrospective review of all thyroidectomy/parathyroidectomy operations performed in a single institution from 1st January 2006 to 31st December 2016. All the operations were performed by consultant endocrine surgeons or by their fellows/registrars under direct supervision. We included in the study all patients that had unequivocally hoarse voice upon extubation. Patients with a preoperative VC palsy documented on laryngoscopy, and/or patients with a follow-up (FU) less than 12 months and/or lost to FU were excluded. Due to the retrospective nature of the study and given that no intervention/change in treatment was performed and that the study will not affect the future management of the patients, the R&I Office of our institution approved this study and the patient’s personal data have been secured.
Operative reports and the patient’s charts were reviewed to collect information regarding the demographics (sex and age at date of operation), the clinical characteristics and the operative details. Pathology reports were screened to confirm the preoperative diagnosis and the presence/characteristics of differentiated thyroid cancer (DTC) were extracted from the pathology reports. The American Joint Committee on Cancer (AJCC) 7th Edition/TNM Classification System for Differentiated Thyroid Carcinoma (DTC) was used for the classification of DTC’s in this study (11). Clinic letters were reviewed to establish the long-term voice outcomes and patients were contacted directly whenever possible to obtain supplementary information.
Local protocol
All patients underwent a neck ultrasound (US) and a fine-needle aspiration cytology (FNAC) was performed whenever a nodule with suspicious feature on US scan was encountered. In case of malignancy, further preoperative staging was performed with computed tomography (CT)/magnetic resonance imaging (MRI) scans. All patients were adequately prepared preoperatively to ensure normal serum levels of thyroid hormones (euthyroid status). Patients that underwent total thyroidectomy routinely stayed overnight with a blood test for levels of serum calcium and parathyroid hormone the next morning at 08:00 am and hospital discharge was on a case-by-case basis. Thyroid hormone replacement was started on day 1 after the operation. Parathyroidectomy patients are operated as day-cases, except for patients with significant comorbidities, living far away and whenever significant postoperative calcium drop is expected. All patients are seen within 2 weeks after the operation in the Outpatients Department for a FU visit and long-term FU is arranged in case of malignancy or complications.
Quality of voice assessment
All patients that have preoperative voice changes and/or have underwent a neck operation, are sent for a formal preoperative laryngoscopy. If in the post-operative period a patient is noted to have altered voice, then a case-to-case plan is formulated and the patient undergoes formal laryngoscopy based on the severity of the hoarseness and associated symptoms (swallowing difficulties, shortness of breath, suspected mechanism of injury). Further referral to Speech and Language Therapy (SLT) is done on a case-to-cases basis. For the purposes of this study, voice outcomes were classified in 3 broad categories; normal, moderate hoarseness and hoarse. We defined persistent RLN palsy as any patient with an immobile VC or clinically significant hoarseness at 6 months post-operation.
Surgical technique of thyroidectomy
Total thyroidectomy was based on the principle of capsular dissection with removal of all thyroid tissue (including pyramidal lobe where present) and with every effort made to identify and preserve all four parathyroid glands. The RLN is identified after mobilising the superior pole of the thyroid and its entire course up to the entry of the larynx is tracked to ascertain its structural integrity. We do not use any energy devices during our operations and all bleeding points are controlled with ties and ligaclips. We avoid the use of monopolar diathermy near the vicinity of the RLN. Our Unit does not use intraoperative neuromonitoring.
Statistics
Statistical analysis was done using the SPSS software (SPSS 20, Chicago, IL, USA). Descriptive statistics were expressed as frequencies and percentages for categorical and as medians with range (min–max) for continuous variables. Data collection and analysis of the results was performed with adherence to data protection principles.
Results
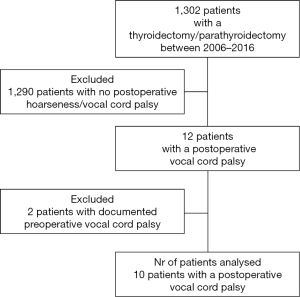
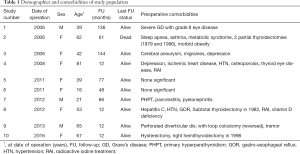
Ten patients fitted the inclusion criteria and were analysed. Figure 1 presents the flowchart of the patients included in the study and the inclusion/exclusion criteria. Table 1 presents the demographic characteristics, follow-up and the preoperative comorbidities of our cohort. The median age at date of operation was 47.5 years (range, 16–81 years) and the M:F ratio was 1:2.3 (M:3, F:7). The median FU was 62.5 months (range, 12–144 months).
Full table
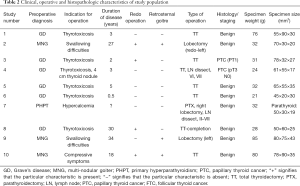
Table 2 presents the clinical, operative and histopathologic characteristics of study population. Patient 7 had an oncologic operation (thyroid lobectomy, parathyroidectomy and lymph node dissection for a presumed parathyroid cancer but the histology revealed a benign parathyroid adenoma). Two out of the 10 patients had a histological diagnosis of malignancy (patients 3 and 4).
Full table
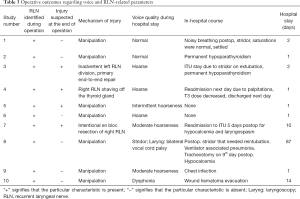
Table 3 presents the operative outcomes, the mechanism of nerve injury and the course of the patients during their hospital stay. There were 7 injuries by manipulation, 1 case of RLN resection, 1 inadvertent division (with primary nerve repair) and 1 RLN was shaved off the thyroid. Patient 8 needed a tracheostomy post-operatively for stridor. The median hospital stay was 1.5 days (range, 1–87 days).
Full table
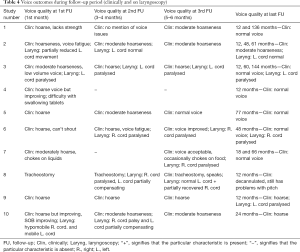
The voice of 3/7 patients with an RLN manipulation injury recovered completely in the long-term while another 3/7 patients had medium-quality voice at long-term (Table 4 and Figure 2). There was 1 patient with an RLN manipulation injury that ended up with a hoarse voice post-operatively (patient 9). The long-term voice outcome of patient 4 was excellent while the 2 patients with RLN resection and inadvertent division ended up with moderately hoarse voice.
Full table
Four out of the 10 patients had permanent VC palsy in the long-term (patients 3, 6, 7, and 9) and their voice outcomes varied; 1 patient had a normal voice, 2 patients had moderate hoarseness and 1 patient had persistent hoarseness.
All patients in this series underwent SLT sessions at different time intervals after their operation. None of the patients received any further surgical interventions during their FU (thyroplasty, etc.).
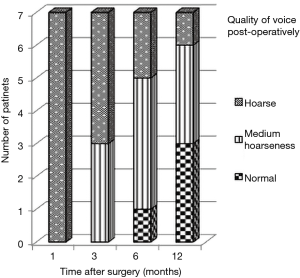
Figure 3 presents the graphical representation of quality of voice (normal, medium hoarseness, hoarse) correlated to timing after surgery only for patients that had RLN injury due to manipulation (n=7). At 12 months post-operatively 3/7 patients had a normal voice, 3/7 had moderate hoarseness and 1/7 was hoarse.
Cases description
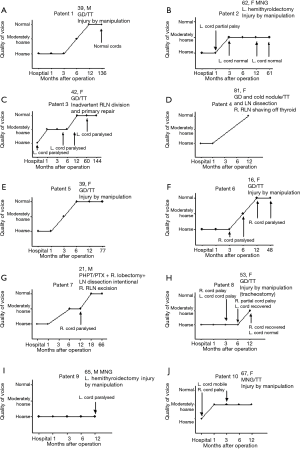
Patient 1 had RLN palsy due to manipulation and eventually made a full recovery phonetically (Figure 2). Patient 2 also had initial RLN palsy due to manipulation (normal VC movement on laryngoscopy at 4 years post-operatively) but remained dysphonic with swallowing difficulties for food and fluids. However, this was attributed to her comorbidities; obesity, COPD, long-term breathing difficulties (the latter was present also pre-operatively) and was eventually diagnosed by SLT with functional dysphonia.
Patient 3 had an inadvertent left RLN injury that was recognised intraoperatively and a primary end-to-end repair of the left RLN was performed with prolene stiches. On extubation, the patient had stridor, a fibreoptic laryngoscopy showed VCs prolapsing on inspiration and the patient was transferred to the ITU for monitoring. The patient’s voice recovered to baseline at 1 year post-operatively despite laryngoscopy showing permanent paralysis of the left VC.
Patient 4 had RLN shaving off the thyroid and the voice recovered completely by 12 months post-operatively. Patient 5 had RLN palsy due to manipulation and the voice recovered fully at 6 months post-operatively. Patient 6 had RLN manipulation injury and permanent cord paralysis with good voice at long-term albeit with occasional episodes of laryngospasm.
Patient 7 had intentional RLN excision due to suspicion of parathyroid cancer and had a planned delayed extubation at 48 hours after the operation. The long-term voice outcome was moderate hoarseness with a paralysed right VC. Patient 8 had an RLN injury due to manipulation with temporary paralysis of VCs (1 complete/1 partial) and stridor. Persistent supraglottic swelling and the risk of bilateral nerve neurapraxia led to an early elective tracheostomy that led to long-term moderate hoarseness despite decannulation and full recovery of both VCs. Patient 9 had persistent hoarseness with VC paralysis at 12 months due to RLN manipulation injury and hence was referred to ENT to discuss the options of a surgical intervention (augmentation, thyroplasty). Patient 10 had long-term (at 24 months) moderate hoarseness due to RLN manipulation injury.
Discussion
In this study we have reviewed our institutional experience with patients presenting with significant post-operative hoarseness following a thyroidectomy and/or a parathyroidectomy. The patients in our cohort had a varied etiology leading to RLN paresis/palsy and the vast majority exhibited improvement in the quality of their voice within the first 6 months.
Up to now, there is still no consensus on the need for a VC assessment prior to thyroid surgery, neither first-time nor re-operative. In the latest BAETS report, the rates of pre-operative laryngoscopy for 1st time thyroid surgery is around 70% and increases to more than 80% for re-operative surgery (3). However, in the post-operative period the percentage of laryngoscopy in the BAETS report falls to 20% (12). This makes accurate assessment of the rate of RLN injury/palsy a challenging problem compounded by high rates of missing data and the lack, until the last revision of the database in October 2014, of a defined time interval by which any cord palsy could be assigned as persistent.
Aside from the lack of trained personnel, a number of other issues such as equipment logistics issues and restraints of time-slots in outpatient appointments can hinder implementation of routine pre-operative laryngoscopies. The policy of our unit is to perform pre-operative laryngoscopy only in re-operative cases and when there is clinically observed hoarseness. The authors acknowledge that subjective assessment of VC paralysis by clinicians could be inaccurate as demonstrated in a study in 2008 by Hanna et al. (13). To complicate things further, it has been shown that vocal recovery from unilateral VC paralysis can occur without the recovery of normal vocal fold motion due to synkinetic reinnervation (restoration of vocal fold muscle tone and bulk, and thus glottic competence during phonation) (12,14,15).
The management of patients with a post-operative VC palsy remains on a case-to-case basis. The key factors influencing the management of such patients are the severity of symptoms (dysphonia, aspiration, dysphagia), the impression of the operating surgeon about the mechanism of the nerve injury (temporary/permanent) and patient characteristics (age, comorbidities, personal preferences). It is not our practise to administer calcium channel blockers in post-operative patients with VC palsy due to the limited amount of evidence in the literature (16-19). In our institution, if the RLN injury is presumed to be temporary, we usually adopt a watch-and-wait approach by following up the patient in the Outpatient’s Clinic up to a period of 1 year. At that point, if there is persistent symptomatology, we refer the patient to an ENT specialist to discuss further surgical options (vocal fold injection, thyroplasty, reinnervation).
RLN injury can be caused by a number of factors such as inadvertent division, traction injury, diathermy injury, etc. (1,10). In our cohort of patients, we had 1 patient that had an inadvertent RLN division that was recognised intraoperatively and the RLN was repaired in an end-to-end fashion with acceptable, but less than perfect, long-term voice outcomes. Similar experience to ours has been previously reported by Enomoto et al., 5 patients with accidental RLN amputation and end-to-end RLN anastomosis had persistent hoarseness at the end of the FU period (7).
It is known that after direct anastomosis of the injured RLN, nerve fibers do regenerate, but this regeneration occurs in a misdirected fashion among adductor fibers and abductor fibers and as a result the reinnervated VC are usually fixed at the median (20,21). However, primary RLN anastomosis is beneficial for the patient as the VC recover from atrophy, and tension during phonation is restored (5,20). Direct anastomosis is possible only if the defect of nerve segment is very short; in all other cases nerve grafting or ansa-to-recurrent nerve anastomosis is advisable (20,22). Miyauchi et al. in 2009 reported that at 1 year after operation, patients with RLN reconstruction had values of maximum phonation time similar to those of normal subjects (20). Yoshioka et al. in 2016 reported that approximately 90% of patients who needed resection of the RLN achieved phonatory recovery following RLN reconstruction albeit there was a 10% with insufficient recovery in phonation (23).
We have classified 7 patients as having RLN palsy due to nerve manipulation based on the fact that the RLN was structurally intact at the end of the operation and that we did not use any energy devices or diathermy in their vicinity. RLN paralysis in these patients with anatomically intact nerve probably occurs due to excessive manipulation and stretching of the nerve, damage to the delicate vasa nervorum and oedema (neuropraxis) (19,24). Interestingly, in our cohort the voice of 3/7 patients with an RLN manipulation injury recovered completely in the long-term while another 3/7 patients had medium-quality voice at long-term. In these 6/7 patients clinical improvement of their voice spanned as early as 1 month post-operatively to up to 6 months after the operation. These data are in agreement with previous studies that report that most RLN palsies that do not involve RLN division resolve spontaneously within 12 months after surgery (7,25,26). Enomoto et al. reported that for the 40 patients in their study 69% of RLN palsies had recovered by 3 months after surgery; 84% had recovered by 6 months after the surgery (7).
The currently available tools to guide VC palsy management in the post-operative period, include the use of laryngeal electromyography which can provide prognostic information on ultimate VC fold mobility in a post-operative patient with VC paralysis (27-29). New techniques and interventions are currently being evaluated or tested in animals in an effort to improve RLN regeneration and voice outcomes including viral gene and stem cell therapies to promote nerve generation (30-34).
The study has a number of limitations including its retrospective nature. Voice changes after thyroidectomy are not uncommon, and are not always related to RLN palsy (3,35). Given that we did not perform preoperative laryngoscopy in all of the patients of our cohort, we can’t exclude the possibility that there was preoperative VC palsy, although this is unlikely given the lack of pre-operative hoarseness or other relevant symptomatology. Furthermore, other well-known risk factors of post-operative VC palsy such as VC trauma from intubation or retraction of the strap muscles in the neck, also cannot be excluded (no patient in our cohort had an uneventful endotracheal intubation) (5).
It has been previously reported that symptoms related to VC paralysis are proportional to the degree of glottic insufficiency; an immobile VC resting close to midline may not produce significant dysphonia (36). It is possible that the incidence of VC palsy presented in this study is an underestimate and some cases of very transient VC paralysis went by undetected as they resulted in very subtle and quick resolving symptomatology.
The authors acknowledge that the mechanism of injury to the RLN for each of the patients presented in this study is as accurate as possible (especially for the ones classified as manipulation injury) given that none of our patients had a surgical re-exploration of their neck to assess the RLN integrity. Furthermore, injury to the superior laryngeal nerve (SLN) is very common in thyroid operations and can occur in up to 40% of patients. However, the assessment of the functional status of the SLN is challenging and not always possible even in units where routine neuromonitoring is implemented. The co-occurrence of SLN and RLN injury in our cohort patients can’t be excluded, although every effort was taken intraoperatively to visually identify and preserve the SLN.
Further research in this field is needed and only multi-institutional collaborations can result in cohort of patients with significant numbers for statistical analysis. New prospective studies investigating patient reported outcomes after thyroid surgery (ex: ThyVoice study REC: 16/EM/0070) will provide valuable information.
In conclusion, the authors of this study present in this cohort of patients the incidence, management and progress of postoperative VC palsies. It is of utmost importance that referral centres, that usually set the standards of the speciality, report their negative outcomes. Given the rarity of this untoward event, even in experienced hands, this study adds to the body of literature and informs clinicians about the long-term outcomes of these patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: This study has been accepted as Poster presentation in the British Association of Endocrine and Thyroid Surgeons Meeting Glasgow 2018.
Ethical Statement: Ethical approval is not required as this study falls under service evaluation.
References
- Hermann M, Alk G, Roka R, et al. Laryngeal recurrent nerve injury in surgery for benign thyroid diseases: effect of nerve dissection and impact of individual surgeon in more than 27,000 nerves at risk. Ann Surg 2002;235:261-8. [Crossref] [PubMed]
- Bhattacharyya N, Fried MP. Assessment of the morbidity and complications of total thyroidectomy. Arch Otolaryngol Head Neck Surg 2002;128:389-92. [Crossref] [PubMed]
- The British Association of Endocrine & Thyroid Surgeons. Fifth National Audit Report, 2017.
- Mau T, Pan HM, Childs LF. The natural history of recoverable vocal fold paralysis: Implications for kinetics of reinnervation. Laryngoscope 2017;127:2585-90. [Crossref] [PubMed]
- Chen X, Wan P, Yu Y, et al. Types and timing of therapy for vocal fold paresis/paralysis after thyroidectomy: a systematic review and meta-analysis. J Voice 2014;28:799-808. [Crossref] [PubMed]
- Meek P, Carding PN, Howard DH, et al. Voice change following thyroid and parathyroid surgery. J Voice 2008;22:765-72. [Crossref] [PubMed]
- Enomoto K, Uchino S, Watanabe S, et al. Recurrent laryngeal nerve palsy during surgery for benign thyroid diseases: risk factors and outcome analysis. Surgery 2014;155:522-8. [Crossref] [PubMed]
- Page C, Zaatar R, Biet A, et al. Subjective voice assessment after thyroid surgery: a prospective study of 395 patients. Indian J Med Sci 2007;61:448-54. [Crossref] [PubMed]
- Grover G, Sadler GP, Mihai R. Morbidity after thyroid surgery: patient perspective. Laryngoscope 2013;123:2319-23. [Crossref] [PubMed]
- Dzodic R, Markovic I, Santrac N, et al. Recurrent Laryngeal Nerve Liberations and Reconstructions: A Single Institution Experience. World J Surg 2016;40:644-51. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Young VN, Smith LJ, Rosen C. Voice outcome following acute unilateral vocal fold paralysis. Ann Otol Rhinol Laryngol 2013;122:197-204. [Crossref] [PubMed]
- Hanna BC, Brooker DS. A preliminary study of simple voice assessment in a routine clinical setting to predict vocal cord paralysis after thyroid or parathyroid surgery. Clin Otolaryngol 2008;33:63-6. [Crossref] [PubMed]
- Crumley RL. Laryngeal synkinesis revisited. Ann Otol Rhinol Laryngol 2000;109:365-71. [Crossref] [PubMed]
- Wang CC, Chang MH, Wang CP, et al. Prognostic indicators of unilateral vocal fold paralysis. Arch Otolaryngol Head Neck Surg 2008;134:380-8. [Crossref] [PubMed]
- Sridharan SS, Rosen CA, Smith LJ, et al. Timing of nimodipine therapy for the treatment of vocal fold paralysis. Laryngoscope 2015;125:186-90. [Crossref] [PubMed]
- Rosen CA, Smith L, Young V, et al. Prospective investigation of nimodipine for acute vocal fold paralysis. Muscle Nerve 2014;50:114-8. [Crossref] [PubMed]
- Hydman J, Remahl S, Bjorck G, et al. Nimodipine improves reinnervation and neuromuscular function after injury to the recurrent laryngeal nerve in the rat. Ann Otol Rhinol Laryngol 2007;116:623-30. [Crossref] [PubMed]
- Mattsson P, Hydman J, Svensson M. Recovery of laryngeal function after intraoperative injury to the recurrent laryngeal nerve. Gland Surg 2015;4:27-35. [PubMed]
- Miyauchi A, Inoue H, Tomoda C, et al. Improvement in phonation after reconstruction of the recurrent laryngeal nerve in patients with thyroid cancer invading the nerve. Surgery 2009;146:1056-62. [Crossref] [PubMed]
- Ezaki H, Ushio H, Harada Y, et al. Recurrent laryngeal nerve anastomosis following thyroid surgery. World J Surg 1982;6:342-6. [Crossref] [PubMed]
- Su WF, Hsu YD, Chen HC, et al. Laryngeal reinnervation by ansa cervicalis nerve implantation for unilateral vocal cord paralysis in humans. J Am Coll Surg 2007;204:64-72. [Crossref] [PubMed]
- Yoshioka K, Miyauchi A, Fukushima M, et al. Surgical Methods and Experiences of Surgeons did not Significantly Affect the Recovery in Phonation Following Reconstruction of the Recurrent Laryngeal Nerve. World J Surg 2016;40:2948-55. [Crossref] [PubMed]
- Zabrodsky M, Boucek J, Kastner J, et al. Immediate revision in patients with bilateral recurrent laryngeal nerve palsy after thyroid and parathyroid surgery. How worthy is it? Acta Otorhinolaryngol Ital 2012;32:222-8. [PubMed]
- Tessema B, Roark RM, Pitman MJ, et al. Observations of recurrent laryngeal nerve injury and recovery using a rat model. Laryngoscope 2009;119:1644-51. [Crossref] [PubMed]
- Lee JC, Breen D, Scott A, et al. Quantitative study of voice dysfunction after thyroidectomy. Surgery 2016;160:1576-81. [Crossref] [PubMed]
- Munin MC, Heman-Ackah YD, Rosen CA, et al. Consensus statement: Using laryngeal electromyography for the diagnosis and treatment of vocal cord paralysis. Muscle Nerve 2016;53:850-5. [Crossref] [PubMed]
- Rickert SM, Childs LF, Carey BT, et al. Laryngeal electromyography for prognosis of vocal fold palsy: a meta-analysis. Laryngoscope 2012;122:158-61. [Crossref] [PubMed]
- Smith LJ, Rosen CA, Munin MC. Vocal fold motion outcome based on excellent prognosis with laryngeal electromyography. Laryngoscope 2016;126:2310-4. [Crossref] [PubMed]
- Choi JS, Oh SH, An HY, et al. Functional regeneration of recurrent laryngeal nerve injury during thyroid surgery using an asymmetrically porous nerve guide conduit in an animal model. Thyroid 2014;24:52-9. [Crossref] [PubMed]
- Zealear DL, Mainthia R, Li Y, et al. Stimulation of denervated muscle promotes selective reinnervation, prevents synkinesis, and restores function. Laryngoscope 2014;124:E180-7. [Crossref] [PubMed]
- Rubin AD, Hogikyan ND, Oh A, et al. Potential for promoting recurrent laryngeal nerve regeneration by remote delivery of viral gene therapy. Laryngoscope 2012;122:349-55. [Crossref] [PubMed]
- Wang B, Yuan J, Chen X, et al. Functional regeneration of the transected recurrent laryngeal nerve using a collagen scaffold loaded with laminin and laminin-binding BDNF and GDNF. Sci Rep 2016;6:32292. [Crossref] [PubMed]
- Lerner MZ, Matsushita T, Lankford KL, et al. Intravenous mesenchymal stem cell therapy after recurrent laryngeal nerve injury: a preliminary study. Laryngoscope 2014;124:2555-60. [Crossref] [PubMed]
- Pei YC, Li HY, Chen CL, et al. Disease Characteristics and Electromyographic Findings of Nonsurgery-Related Unilateral Vocal Fold Paralysis. Laryngoscope 2017;127:1381-7. [Crossref] [PubMed]
- Sulica L. The natural history of idiopathic unilateral vocal fold paralysis: evidence and problems. Laryngoscope 2008;118:1303-7. [Crossref] [PubMed]