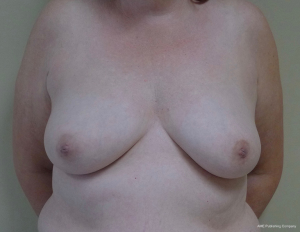
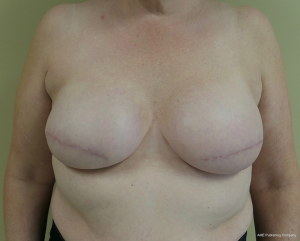
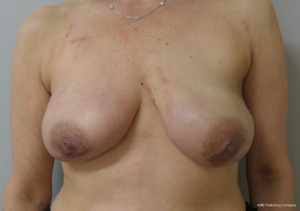
Two-stage prepectoral breast reconstruction
Introduction
Prosthetic breast reconstruction continues to remain the primary type of reconstruction offered to women following mastectomy. The reasons for this include relative simplicity, ease of technique, and rapid recovery relative to autologous reconstruction. In addition, aesthetic outcomes range from good to excellent in the majority of patients with low and acceptable complication rates. Prosthetic reconstruction can be performed in one or two stages, with approximately 80% of patients and surgeons opting for the two-stage approach in which a partially filled tissue expander is placed first followed a few months later by exchanging the tissue expander for a permanent implant (1). The benefits of this approach include the ability to offload pressure on the skin flaps following mastectomy and the opportunity to select the ideal permanent implant following the expansion phase. The two-stage approach is advocated as more predictable, reproducible, and resulting in fewer unplanned operations due to size change and malposition.
The past decade has been a time for many innovations and advancements with prosthetic reconstruction that include nipple sparing mastectomy, autologous fat grafting, the use of acellular dermal matrix (ADM), and better prosthetic devices (2-7). From a historical perspective, the majority of prosthetic devices have been placed in the dual plane position where the upper portion of the device is under the pectoralis major and the lower portion of the device is under the lower mastectomy skin flap, but separated from it with ADM. This has been successful in many patients; however, one of the limitations of the dual plane or partial muscle coverage technique is the animation deformity that occurs with pectoralis major muscle contraction as well as muscle spasm that have been demonstrated to occur in approximately 80% of patients (8). As an alternative to partial or total subpectoral placement, the option of prepectoral placement of prosthetic devices has come to fruition (9-19). The benefits of prepectoral placement include elimination of animation deformity, elimination of muscle spasm, and optimal positioning of the device on the chest wall in accordance with the medial border of the mastectomy, rather than the medial origin/border of the pectoralis major muscle (8,19,20).
This manuscript will review the authors approach in terms of patient selection, surgical technique and review outcomes with 2-stage prepectoral breast reconstruction following mastectomy. It will be based on published manuscripts focused on comparative analyses between prepectoral and partial subpectoral prosthetic reconstruction.
Patient selection
Patient selection is an important factor when considering prosthetic breast reconstruction and especially with prepectoral reconstruction (4,21-24). In general, patients should be in good general health without significant co-morbidities. In patients with diabetes mellitus, optimization of blood glucose and HbA1c is essential in order to reduce the incidence of delayed healing and incisional dehiscence. The use of tobacco products is prohibited for 1-month prior to and following mastectomy to reduce the incidence of delayed healing and mastectomy skin flap necrosis that may lead to reconstructive failure. Patients that have had prior breast conservation with radiation therapy should be informed that adverse events and reconstructive failure could also be more likely (25).
When considering prepectoral, two-stage prosthetic reconstruction, patient expectations must be adequately assessed and understood (21). It is important to discuss why the two-stage approach is beneficial for the previously mentioned reasons. Some surgeons and patients will consider direct to implant reconstruction, especially with the advent of nipple sparing mastectomy; however, there are several caveats to this approach. First, not all patients are candidates for nipple sparing and in some patients, skin and nipple resection is necessary; thus, increasing the need for tissue expansion. Direct to implant reconstruction can be considered in women with small to moderate breast volume that typically ranges in cup size from A to C. However, as breast size increases to D or larger, direct-to-implant reconstruction has been demonstrated to result in a higher revision rate (26).
Another factor, and arguably the most important when considering prepectoral reconstruction, is the quality of the skin flaps following mastectomy. It is essential that the skin flaps be well perfused prior to proceeding with prepectoral reconstruction. Tissue perfusion can be assessed using various devices such as fluorescent angiography, hyperspectral imaging, or clinically by demonstration of arterial and venous bleeding at the skin edges (27,28). When mastectomy skin flap perfusion is compromised, many surgeons now prefer the delayed-immediate approach whereby the mastectomy skin flaps are placed on the chest wall and the incision is closed without reconstruction (29). The reconstruction is delayed for 2–3 weeks based on the viability of the mastectomy skin flaps. Other reconstructive options would include partial or total muscle coverage of the tissue expander; however, this option is less frequently performed to avoid the complication of animation, muscle spasm, and patient dissatisfaction. In some patients, the mastectomy skin flaps may be thin but are well perfused. In this situation, most surgeons will recommend two-stage reconstruction using a minimally or partially inflated tissue expander to offload the pressure on the skin flaps.
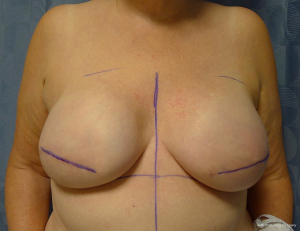
Device selection is based on the principles of bio-dimensional planning and includes the preoperative assessment of the base diameter of the breast as well as other relevant measurements to assess breast symmetry such as the transverse distance between sternum to nipple and the vertical distance form sternal notch to nipple (5-7). If possible, an estimation of breast volume, soft tissue compliance, breast ptosis, and body habitus will be useful. Tissue expander options will depend upon the manufacturers that make these devices and surgeons preference. The authors’ preference is to use tabbed tissue expanders to precisely secure the device to the chest wall and to minimize the risk of displacement or malposition (30).
Operative technique
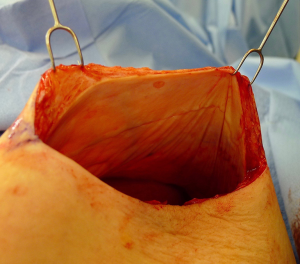
There are several options for two-stage prepectoral reconstruction that include the use of ADM or not. Most surgeons will use an ADM for reasons that include soft tissue support for the mastectomy skin flaps, compartmentalization of the prosthetic device, lessening the inflammatory response associated with prosthetic devices and reducing the incidence of capsular contracture (31,32). However, some surgeons feel that ADM use is unnecessary in all cases, so there is some degree of controversy in this regard (15). This manuscript will focus of prepectoral 2-stage tissue expanders using ADM because this is the authors’ technique based on currently available evidence. There are on-label and off-label techniques for the use of ADM that will be reviewed. Figures 1-8 illustrate a patient following bilateral skin sparing mastectomy with immediate reconstruction using tissue expanders and implants placed in the prepectoral position.
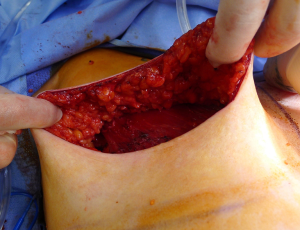

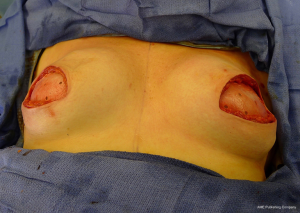
ADM assembly
ADM can be used to provide anterior cover only, anterior and partially posterior or complete anterior and posterior coverage of the device (33). As such, the size of ADM used will vary between surgeons. Options for ADM include a 16×20 sheet or two rectangular or contoured sheets sewn together to make a larger sheet. Perforations or fenestrations in the ADM are highly recommended to promote adherence of the ADM to the mastectomy skin as well as to allow the early movement of fluid so that the risk of a seroma between the ADM and the mastectomy skin flap is minimized.
Two-stage prepectoral reconstruction with ADM (on-label)
The on-label use of ADM for prepectoral reconstruction is based on using the ADM to provide soft tissue support to the mastectomy skin flaps without wrapping the ADM around the device. This technique will be reviewed in a step-by-step basis.
- The mastectomy skin flaps are assessed for perfusion and thickness.
- The mastectomy pocket is irrigated to remove blood and fat droplets and the skin is reprepped and draped to ensure a clean environment.
- The ADM is placed in the mastectomy pocket with the dermal side oriented toward the mastectomy skin flaps and the basement membrane side towards the device.
- The tissue expander can be partially filled with air or saline depending on surgeon preference. The author prefers to use air for several reasons that include even distribution throughout the expander, lightweight and resulting in less suture pull-through from the fragile muscle when suturing the expander tabs to the pectoralis major muscle.
- With the on-label technique, the ADM is used to provide tissue support for the mastectomy skin flaps and overlies the entire anterior surface of the tissue expander with a short lower pole cuff.
- Skin sparing mastectomy.
- An inferiorly based cuff of ADM is created at the level of the inframammary fold such that approximately 2–3 cm of ADM is placed on the chest wall to provide inferior support for the tissue expander;
- The ADM is sutured to the chest wall medially and superiorly with absorbable sutures creating a pocket for tissue expander insertion;
- The tissue expander is positioned and oriented into the newly created ADM pocket such that the device is anchored at the true medial border of the pocket and along the desired inframammary fold;
- The tabs of the tissue expander are sutured to the chest wall using an absorbable suture;
- The lateral aspect of the ADM is sutured to the chest wall using an absorbable suture;
- Nipple sparing mastectomy.
- The superior aspect of the ADM is sutured to the upper aspect of the desired tissue expander location using absorbable sutures. This sometimes correlates with the upper border of the mastectomy but not always;
- The ADM is sutured to the medial and lateral borders of the mastectomy space to correlate with the dimensions of the tissue expander;
- A suture is placed on the upper tab of the tissue expander and then parachuted into the mastectomy pocket and sutured t the pectoralis major muscle;
- The medial and lateral tabs of the tissue expander and sutured to the pectoralis major or the fascia at the level of the desired inframammary fold;
- The inferior edge of the ADM is then tucked under the tissue expander to create a 2–3 cm cuff to provide inferior tissue support.
- The periprosthetic space is copiously irrigated with an antibiotic containing irrigation solution.
- One or two drains are inserted and directed around the perimeter of the device between the ADM and the mastectomy skin as well as towards the axilla if necessary.
- The incision is closed in layers and an incisional dressing is applied.
Two-stage prepectoral reconstruction with ADM (off-label)
- The desired tissue expander and ADM construct are obtained and placed in an irrigation solution.
- On the back table, the ADM is draped around the expander with the dermal surface facing outward (eventually towards the mastectomy skin flap).
- The tissue expander—ADM construct is then flapped upside-down and posterior spanning sutures are placed from one edge of the ADM to the edge directly opposite edge with a soft absorbable suture. This is continued in a pinwheel fashion until the ADM—tissue expander construct is secure. This constitutes the partial wrap technique because the posterior surface of the tissue expander remains in direct contact with the pectoralis major muscle.
- Some surgeons prefer to completely wrap the tissue expander in which case the ADM is in direct contact with the entire pectoralis major muscle and the mastectomy skin flap.
- The tabs of the tissue expander are exteriorized to facilitate suturing to the pectoralis major muscle. The construct is now ready for insertion.
- The mastectomy skin flaps are assessed for perfusion and thickness.
- The mastectomy pocket is irrigated to remove blood and fat droplets and the skin is reprepped and draped to ensure a clean environment.
- Skin sparing mastectomy: the tissue expander-ADM construct is inserted into the pocket, properly positioned and oriented, and then sutured via the three tabs to the pectoralis major muscle or fascia using absorbable sutures.
- Nipple sparing mastectomy: a suture is placed in the upper tab that is also placed at the upper border of the desired location of the tissue expander. The tissue expander-ADM construct is then parachuted into the mastectomy space through the inframammary incision and the suture is ligated. Once positioned the remaining two tabs along the infero medial and lateral aspect of the tissue expander are sutured to the chest wall.
- The periprosthetic space is copiously irrigated with an antibiotic containing irrigation solution.
- One or two drains are inserted and directed around the perimeter of the device between the ADM and the mastectomy skin as well as towards the axilla if necessary.
- The incision is closed in layers and an incisional dressing is applied.
Results
Outcomes following two-stage prepectoral prosthetic reconstruction have been favorable with several studies confirming that there is no difference in the rate of adverse events when compared to the dual plane or partial subpectoral approach. Tables 1-4 summarize the demographics and outcomes of five manuscripts that directly compare results of two-stage prepectoral versus to-stage partial subpectoral prosthetic breast reconstruction (11,12,15,16,19). In reviewing the demographics, several noteworthy observations can be made. An acellular matrix was used in all studies except the Salibian study. The majority of mastectomies in these cohorts were nipple sparing that corroborates recent trends in mastectomy preference. The majority of patients in all five studies had a BMI <30 and did not use tobacco products confirming the importance of patient selection. With regard to adverse events, the incidence of surgical site infection (prepectoral range: 2.4–8.1%, partial subpectoral range, 4.8–11.5%) and seroma (prepectoral range, 3.6–15%, partial subpectoral range, 2.4–6.5%) were similar with minor variations between studies in both the prepectoral and the partial subpectoral cohorts. The rate of device explantation resulting in reconstructive failure was increased in all studies when the device was placed in the partial subpectoral position (range, 4.3–15.4%) compared to the prepectoral position (range, 1.2–8.5%). Unfortunately, the incidence of capsular contracture could not be adequately assessed from these studies because the length of follow-up was too short; however, in the Sbitany paper, a comparison of capsular contracture between radiated and non-radiated patients demonstrated no capsular contracture in the non-radiated cohort and a 14.3% and 17.6% incidence of skin tightening in the prepectoral cohort and partial subpectoral cohorts respectively (11). It should be noted that the appearance of capsular contracture between the prepectoral and partial subpectoral cohorts are different because superior migration of the device occurs when the device is placed in the subpectoral space whereas there is minimal superior migration when the device is placed in the prepectoral space.

Full table

Full table
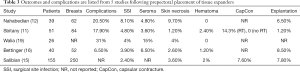
Full table
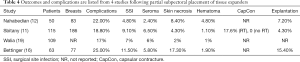
Full table
Patient reported outcomes were assessed in two studies. Walia et al. compared patient satisfaction following prepectoral and partial subpectoral tissue expander reconstruction and demonstrated improved satisfaction in the prepectoral cohort (19). In the prepectoral cohort, 55% of patients reported that they were satisfied with the breast outcome and 88% of patients reported that they were satisfied with the overall outcome. This is in contrast to the partial subpectoral cohort where 47% of patients were satisfied with the breast outcome and 75% were satisfied with the overall outcome. In the Salibian study, 84.2% of patients reported good to very good outcomes in the setting of no radiation (15). This was reduced to 76.4% of patients when the radiation was delivered following tissue expander placement and 64.2% of patients when the radiation was delivered premastectomy.
Discussion
Two-stage prepectoral reconstruction using a tissue expander followed by an implant confers several advantages compared to one stage. By performing the reconstruction in two stages the surgeon can optimize permanent implant selection by selecting a device that will ideally fit the periprosthetic space and meet the patients expectation. This is especially important with prepectoral reconstruction because a hand-in-glove fit is critical to ensure that the implant does not shift or flip. Another advantage of the two-stage approach is that by placing a partially filled tissue expander versus a prefilled permanent implant, the surgeon can offload pressure on the mastectomy skin flaps and reduce the risk of mastectomy skin flap necrosis. By maintaining a low volume and weight initially, the gravitation effect of the device during the early phases of wound healing will be less and may result in less device malposition and displacement.
An important consideration and point of controversy is whether prepectoral implant reconstruction should be performed with the use of an ADM or not. The issue is primarily focused on the cost of the material, not its performance, because the role and benefit of ADM with prepectoral reconstruction is amplified. The ADM provides tissue support for the mastectomy skin flaps and it also compartmentalizes the device to maintain its position on the chest wall. It is important to remember that following mastectomy, all of the retaining ligaments of the breast (coopers ligaments) are removed. Implants placed in a purely subcutaneous pocket will have no support other that what is provided by the skin flaps and the ensuing capsule that forms. The ADM will serve as a hammock for the tissue expander and the implant and maintain the position of the device over time. This has been noted in the authors’ personal experience with 3-year follow-up to date (unpublished data). In addition, the ADM tends to reduce the inflammatory response associated with wound healing and the use of a foreign body such as a breast tissue expander or implant (32,34). The benefits of ADM in reducing capsular contracture with devices placed in the subpectoral position have been well documented (31). Studies of prepectoral placement of devices without ADM have demonstrated higher rates of capsular contracture (15,35) compared to when ADM is used (18,36).
The topic of capsular contracture associated with prosthetic devices deserves discussion. The classic definition of capsular contracture is based on the appearance of the breast with class 1 being normal, class 2 being firm without visible distortion, class 3 being firm and distorted, and class 4 being all of the above including pain (37). This can occur when devices are placed in the prepectoral or subpectoral positions. It is well known that radiation therapy will increase the rate of capsular contracture, especially when devices are placed in the partial subpectoral plane (38). The reason for this is that when the inferior origin of the pectoralis major muscle has been divided, one of the effects of the radiation is to cause muscle retraction and fibrosis resulting in the upward displacement of the implant due to the pull of the muscle. Interestingly, this effect is eliminated with prepectoral placement of devices because the inferior origin of the muscle is not divided; therefore, muscle retraction does not occur and cephalad displacement of the expander or implant does not occur (39). Figures 9-11 illustrate the preoperative, early postoperative prior to radiation, and later postoperative following radiation appearance of a patient to illustrate the point. The other effect of radiation is to cause skin tightening/fibrosis and loss of elasticity resulting in a breast that usually does not descend over time. Based on these observations, prepectoral placement of tissue expanders is now preferred when radiation therapy is planned.
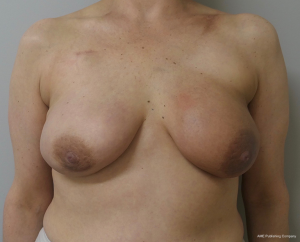
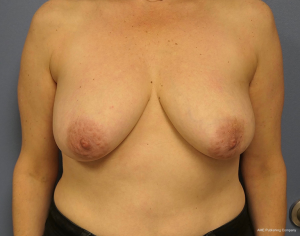
Conclusions
In summary, two-stage prepectoral reconstruction is performed in the majority of patients following mastectomy. The operation is technically simple, fast, and usually associated with less pain and recovery time. The rate of complications is similar to that of partial subpectoral reconstruction. ADM use is recommended to provide tissue support following mastectomy and to provide long-term stability to the reconstruction itself. Prepectoral reconstruction is considered in patients who will receive post mastectomy radiation therapy and may be associated with improved aesthetic and clinical outcomes. Further study will be necessary to confirm the long-term success of prepectoral breast reconstruction.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Nahabedian is a consultant for Allergan (Irvine CA, USA) and Chief Surgical Officer for PolarityTE (Salt Lake City, UT, USA). Dr. Jacobson is a consultant for Allergan (Irvine, CA, USA). No assistance (financial or otherwise) was provided in preparation of this manuscript.
Informed Consent: All patients have signed a consent for using photographs and images for education purposes.
References
- Available online: ASPS statistics. Accessed August 16, 2018.www.plasticsurgery.org
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [Crossref] [PubMed]
- Endara M, Verma K, Chen D, et al. Breast reconstruction following nipple spring mastectomy: A systematic review of the literature with pooled analysis. Plast Reconstr Surg 2013;132:1043-54. [Crossref] [PubMed]
- Nahabedian MY. Acellular Dermal Matrices in Primary Breast Reconstruction: Principles, Concepts, and Indications. Plast Reconstr Surg 2012;130:44S-53S. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. Bioengineered Breast: Concept, Technique, and Preliminary Results. Plast Reconstr Surg 2016;137:415-21. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. Breast Implant Design. Gland Surg 2017;6:148-53. [Crossref] [PubMed]
- Gabriel A, Maxwell GP. The Evolution of Breast Implants. Clin Plast Surg 2015;42:399-404. [Crossref] [PubMed]
- Becker H, Fregosi N. The Impact of Animation Deformity on Quality of Life in Post-Mastectomy Reconstruction Patients. Aesthet Surg J 2017;37:531-6. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Woo A, Harless C, Jacobson SR. Revisiting an old place: single surgeon experience on post-mastectomy subcutaneous implant-based breast reconstruction. Breast J 2017;23:545-53. [Crossref] [PubMed]
- Sbitany H, Piper M, Lentz R. Prepectoral Breast Reconstruction: A Safe Alternative to Submuscular Prosthetic Reconstruction following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;140:432. [Crossref] [PubMed]
- Nahabedian MY, Cocilovo C. Two-Stage Prosthetic Breast Reconstruction: A Comparison Between Prepectoral and Partial Subpectoral Techniques. Plast Reconstr Surg 2017;140:22S-30S. [Crossref] [PubMed]
- Rebowe RE, Allred L, Nahabedian MY. The Evolution from Subcutaneous to Prepectoral Prosthetic Breast Reconstruction. Plast Reconstr Surg Glob Op 2018;6:e1797. [PubMed]
- Ter Louw RP, Nahabedian MY. Prepectoral breast reconstruction. Plast Reconstr Surg 2017;140:51S-9S. [Crossref] [PubMed]
- Salibian AH, Harness JK, Mowlds DS. Staged Suprapectoral Expander/Implant Reconstruction without Acellular Dermal Matrix following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;139:30-9. [Crossref] [PubMed]
- Bettinger LN, Waters LM, Reese SW, et al. Comparative Study of Prepectoral and Subpectoral Expander-Based Breast Reconstruction and Clavien IIIb Score Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1433. [Crossref] [PubMed]
- Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg 2016;69:e77-86. [Crossref] [PubMed]
- Berna G, Cawthorn SJ, Papaccio G, et al. Evaluation of a novel breast reconstruction technique using the Braxon® acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg 2017;87:493-8. [PubMed]
- Walia GS, Aston J, Bello R, et al. Prepectoral Versus Subpectoral Tissue Expander Placement: A Clinical and Quality of Life Outcomes Study. Plast Reconstr Surg Glob Open 2018;6:e1731. [Crossref] [PubMed]
- Kobraei EM, Cauley R, Gadd M, et al. Avoiding Breast Animation Deformity with Pectoralis-Sparing Subcutaneous Direct-to-Implant Breast Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e708. [Crossref] [PubMed]
- Nahabedian MY. Breast Reconstruction: A Review and Rationale for Patient Selection. Plast Reconstr Surg 2009;124:55-62. [Crossref] [PubMed]
- Nahabedian MY. Prosthetic Breast Reconstruction With Acellular Dermal Matrices: Achieving Predictability and Reproducibility. Plast Reconstr Surg Glob Open 2016;4:e698. [Crossref] [PubMed]
- Sbitany H. Important Considerations for Performing Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:7S-13S. [Crossref] [PubMed]
- Gabriel A, Maxwell GP. Prepectoral Breast Reconstruction in Challenging Patients. Plast Reconstr Surg 2017;140:14S-21S. [Crossref] [PubMed]
- Nahabedian MY. AlloDerm Performance in the Setting of Prosthetic Breast Surgery, Infection, and Irradiation. Plast Reconstr Surg 2009;124:1743. [Crossref] [PubMed]
- Gdalevitch P, Ho A, Genoway K, et al. Direct-to-implant single-stage immediate breast reconstruction with acellular dermal matrix: predictors of failure. Plast Reconstr Surg 2014;133:738e-47e. [PubMed]
- Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 2012;129:778e-88e. [Crossref] [PubMed]
- Mattison GL, Lewis PG, Gupta SC, et al. SPY imaging use in postmastectomy breast reconstruction patients: preventative or overly conservative? Plast Reconstr Surg 2016;138:15e-21e. [Crossref] [PubMed]
- Zenn MR. Staged immediate breast reconstruction. Plast Reconstr Surg 2015;135:976-9. [Crossref] [PubMed]
- Spear SL, Economides JM, Shuck J, et al. Analyzing implant movement with tabbed and nontabbed expanders through the process of two-stage breast reconstruction. Plast Reconstr Surg 2014;133:256e-60e. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Berry C, et al. Acellular Dermal Matrix-Assisted Direct-to-Implant Breast Reconstruction and Capsular Contracture: A 13-Year Experience. Plast Reconstr Surg 2016;138:329-37. [Crossref] [PubMed]
- Basu CB, Leong M, Hicks MJ. Acellular Cadaveric Dermis Decreases the Inflammatory Response in Capsule Formation in Reconstructive Breast Surgery. Plast Reconstr Surg 2010;126:1842. [Crossref] [PubMed]
- Sigalove S. Options in Acellular Dermal Matrix–Device Assembly. Plast Reconstr Surg 2017;140:39S-42S. [Crossref] [PubMed]
- Prantl L, Schreml S, Fichtner-Feigl S., et al. Clinical and Morphological Conditions in Capsular Contracture Formed around Silicone Breast Implants. Plast Reconstr Surg 2007;120:275. [Crossref] [PubMed]
- Hammond DC. Treatment of breast animation deformity in implant-based reconstruction with pocket change to the subcutaneous position. Plast Reconstr Surg 2015;135:1540. [Crossref] [PubMed]
- Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous direct-to-implant breast Reconstruction: Surgical, functional, and aesthetic results after long-term follow-up. Plast Reconstr Surg Glob Open 2016;3:e574. [Crossref] [PubMed]
- Spear SL, Baker JL. Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg. 1995;96:1119-23; discussion 1124. [Crossref] [PubMed]
- Hirsch EM, Seth AK, Fine NA. Capsular Contracture after Breast Reconstruction: A Modified Classification System Incorporating the Effects of Radiation. Plast Reconstr Surg 2012;129:870e-1e. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction and Postmastectomy Radiotherapy: Short-Term Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1631. [Crossref] [PubMed]