The effect of CT angiography and venous couplers on surgery duration in microvascular breast reconstruction: a single operator’s experience
Introduction
The deep inferior epigastric perforator (DIEP) flap is now considered the gold standard in autologous breast reconstruction (1,2). However, well-executed harvesting and successful performance of DIEP flap surgery requires considerable expertise (3), with careful preoperative and intraoperative decision-making being essential. Preoperatively, study of the donor vessels and abdominal wall vascular anatomy has been shown to be helpful in reducing operative times and aiding successful free tissue transfer (4-6). The major abdominal wall vascular perforators and the courses of the main branches of the deep inferior epigastric vessels can be accurately identified with computed tomography angiography (CTA), magnetic resonance angiography (MRA) or Doppler ultrasonography (3,5,7,8). Studies have shown that use of such preoperative road maps can reduce operative times (6,9).
Microvascular anastomosis is one of the critical intraoperative steps of free flap surgery. Most consider the venous anastomosis paramount because of the increased propensity for venous thrombosis with venous insufficiency being the most common cause for return to theatre (10-12). The venous coupler (VC) has been shown to be a quick and reliable method for re-establishing venous drainage with reduced anastomotic failure when compared to hand sewn vessels (13-15).
However, despite the many reports of reduced operative times with the use of CTA (4,16,17) or VCs (5,18) (Table 1), there are none that have compared the effect of these two interventions relative to each other. The principal aim of this study was therefore to review the effect of VCs alongside CTA on the operative times of free flap breast reconstruction (FFBR).
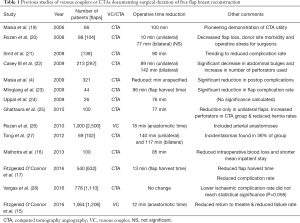
Full table
Methods
All free flap breast reconstructions performed by a single plastic surgeon (CMM) at a tertiary university hospital breast cancer referral centre between August 2008 and February 2014 were included in this retrospective cohort study, to ensure that all patients before and after the introduction of CTA (November 2011) were included. Bipedicled free flaps (n=26) were excluded as was one patient with incomplete data. Patients with neither intervention were compared to those who received only VCs (introduced in June 2010) and those with both interventions. It was not possible to study the effect of CTA without VCs as all CTA patients had had VCs. Patients were identified from a prospectively collected free flap database and the senior author’s free flap logbook. Operations were performed by two teams (one consultant and two assistant surgeons) so recipient vessel exposure was performed at the same time as flap harvest. Details were collected on patient demographics, flap type, ischaemia time, total operative time, number of veins used and coupler size. Flap harvest time was not recorded.
The purpose of including only a single surgeon’s cases was three-fold, firstly to eliminate inter-operator variability, secondly to standardise the internal mammary vessel exposure technique (all total rib preservation) and thirdly to standardise the method of venous anastomoses (exclusive coupler use after June 2010). Operative time was defined as from “knife-to-skin” to insertion of the last stitch (as documented independently by the nursing staff). The surgeon has been in practice for over 10 years so a surgical learning curve is unlikely to be in effect.
A literature search was conducted through PubMed and Web of Science with the terms, “computed tomography angiography”, “breast reconstruction”, “venous coupler”, “operative duration”, “anastomotic time” and “venous anastomoses”. All relevant reports were included (Table 1). Data analysis was completed with IBM SPSS Software Version 24.0 (IBM Corp, 2017. IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp). Data were analysed with the Kolmogorov-Smirnov test and were not found to follow a normal distribution. Therefore, differences in operative and ischaemia times between the groups were evaluated with the nonparametric Mann-Whitney U test. Statistical significance was determined by a value of P≤0.05.
Results
One hundred and twenty patients were divided into three groups; the last 40 patients without intervention (WI), the 40 patients that had received VC only, and the first 40 patients who received CTA with venous coupler (CT-VC). The mean ages were comparable; Table 2 shows characteristics of all three patient groups.

Full table
A total of 143 flaps in 120 patients (mean age =50.5 years; range, 28–68) were included in this study. Ninety-seven patients received unilateral flaps whilst 23 patients had bilateral flaps. Two thirds (65%) of the flaps were performed in the immediate setting at the time of mastectomy. The mean total operative time for all patients was 542.6 minutes (range, 270–840) with a mean ischaemic time of 89.1 minutes (range, 38–137).
Duration of surgery
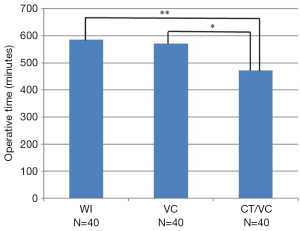
The mean operative times in minutes were 585.5 (r =470–840), 571.6 (r =364–780) and 472.1 (r =270–700) for WI, VC and CT-VC, respectively (Figure 1, Table 3). Introduction of couplers did not significantly reduce the operative time compared to no intervention (572 vs. 586 min, P=0.5306). However, patients with both interventions (CT-VC) had significantly shorter operative times versus WI by 114 minutes (472.1 vs. 585.5 min, P<0.00001). This pattern remained the same when the group was divided in unilateral, bilateral, immediate and delayed flaps (Tables 3,4).

Full table

Full table
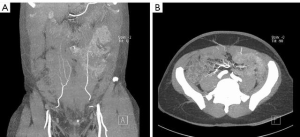
Interestingly, the CT-VC group had a significantly reduced operative time compared to the VC group by 100 minutes (472.1 vs. 571.6 min, P=0.0002), implying that the main factor in the reduction of surgical duration was the introduction of CTA (Figure 1, Table 3). Although this trend was mirrored in unilateral and delayed flaps (P<0.00001 and P=0.0139, respectively), no significance was noted when comparing the VC group with CT-VC in bilateral or immediate flaps (P<0.1141 and P=0.9920, respectively). Figure 2A shows a coronal CTA image of the anterior abdominal wall vessels. Figure 2B shows the corresponding transverse CTA image of the same.
Ischaemia time
The introduction of each intervention showed a stepwise decrease in ischaemia time (Table 2, Figure 3). The use of both modalities significantly reduced the ischaemia time from 100 to 80 minutes when compared to WI (CT-VC vs. WI, P<0.00001). Similarly, CT-VC decreased ischaemia time compared to VC alone (80 vs. 89 minutes, P=0.0307). There was also a significant difference between the ischaemia times of VC patients versus those with no intervention (89 vs. 100 minutes, P=0.0106, Table 4).

Chronological analysis
The flaps were analysed within each category of WI, VC and CT-VC, to assess differences in unilateral flap operative and ischaemia times due to chronology. There was insufficient sample size to perform this analysis on bilateral flaps. Each category was equally divided into two, dependent on their timeline, resulting in “early” and “late” groups for each of the three categories. Results are shown in Table 5.

Full table
Flap survival
All flap transfers were successful and there was no significant difference in the numbers of flaps requiring re-exploration (P=0.2356) or redo of venous anastomoses (P=0.3868).
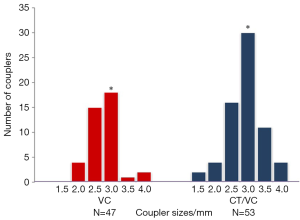
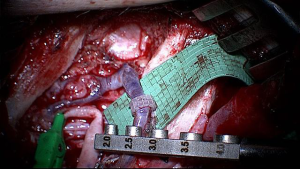
Figure 4 and Table 6 show the distribution of coupler sizes in the VC and CT-VC groups. A greater majority of venous couplers were size 3.0 mm or larger in the CT-VC group (68%) than VC group (53%) (P=0.1317). The median size in VC was 3.0 mm and was 3.0 mm in CT-VC. Figure 5 shows an intraoperative image of a VC.

Full table
Discussion
Although there are numerous reports of the independent benefits of CTA and VC on operative times (4,9,15-17), there have been no studies of effects of VCs alone versus combined with CTA. Our study has demonstrated that CTA is associated with significantly reduced operative time in combination with VCs. The combination shortened the total operating time by almost two hours (114 minutes) compared to no intervention. However, detailed analysis revealed that this decrease was largely due to CTA effect (100 out of the 114-minute reduction in operative time).
Our findings in this study are similar to other reports regarding CTA which have shown reduced operative times ranging from 10 to 140 minutes (4,15-17). This undoubtedly is because it facilitates surgery by providing a preoperative surgical road-map. CTA allows preoperative identification of the most suitable perforators, elaboration on perforator anatomy (location described with reference to the umbilicus, and delineation of the vessel paths including tortuosity and intramuscular course) (30). Thus, an operative “game plan” can be made before surgery. Additionally, it does not involve learning a new surgical technique unlike the use of the coupler and so there is no intraoperative surgical learning curve.
The changes in the ischaemia times mirrored those in the total operative times although CTA appeared to have a greater impact. This was a surprising finding as CT angiography (unlike the venous coupler) does not have a direct impact on the technical aspects of the microanastomosis. One explanation for this could be reduced fatigue and increased confidence in the surgeon due to quicker flap harvest afforded by the CTA allowing a more predictable harvest and an earlier start to the microsurgery when the surgeon is “fresher”. Another explanation is that surgical experience has a positive influence on surgery duration. All operations were conducted by a single operator with more than 10 years in practice, who conducts over 30 free flap breast reconstructions each year. However, there may be still be a learning curve in effect, resulting in a reduction in ischaemia times with time regardless of the intervention. This is however, highly unlikely after 10 years.
In our study, VCs reduced total operative time by 14 minutes, Fitzgerald et al. had similar findings in their study with 12 minutes reduction in anastomotic time which was significant (15,17). However, we acknowledge that the VC use requires adoption of a new surgical technique by the surgeon and thus any reductions in the operative time may well have been ‘averaged out’ by the learning curve (31,32). This is supported by the chronological analysis which demonstrated a significant reduction in operative time for unilateral flaps in the VC early versus VC late. Thus, implying that as the surgeon became more experienced in this new technique, operations became shorter and this effect extended into the CTA introduction period. The lack of statistical significance in the observed reduction of operative time with VCs versus the no intervention group could also be attributed to the small sample size and the “relatively short” time that the VC intervention was employed before CTA was introduced (17 months). Interestingly, the superior effect of CTA compared to VCs appears to be absent in bilateral and immediate flaps. This may reflect the impact of facilitation by CTA which allows the flap to be raised before the mastectomy is completed so flap harvest is no longer the rate-limiting step in immediate flaps. Bilateral reconstruction, which includes an immediate flap, is similarly affected.
Small reductions in operative time in isolation may not be significant but, as this study has shown, when combined with other interventions can amount to significant effects. Perhaps the true benefit of the coupler is not in decreasing ischaemia times but reducing adverse venous events, which lead to potential flap failure or fat necrosis (15,18). In fact, our study showed with the introduction of CTA the average VC size increased. This may well be because the senior surgeon became more experienced with the technique and more confident in using larger sizes, the advantage of this being that for each 0.5 mm increment in coupler diameter there is a four-fold increase in venous flow.
Unlike previous reports (15), our study was confined to a single operator in order to eliminate inter-operator variability and also to standardise the recipient vessel exposure to the total rib preservation technique which has already been shown to reduce operative times with or without coupler use (29,33). Although trainee surgeons were also involved in the operations, the observations above relating to coupler use, CTA effect and combination still hold true.
In free flap breast reconstruction (FFBR), the ultimate goal is successful flap transfer with minimal complications to the patient, in order to shorten post-operative recovery and improve patient outcomes. Shorter anaesthetic and operative times have been shown to reduce post-operative recovery time and improve the overall patient experience (16,34). In addition, the cost saving implications are considerable. Some studies report costs utility savings as high as $3,179, a gain in quality adjusted life-years of 0.25 (9), compared to the $650 cost of CTA (9), and £169.50 cost for a VC (15). The cost of use of an operating theatre is estimated at £14/minute (15) so potential savings are £1,109.60 and £25.10 for CTA and VC, respectively. Hence, the expense of CTA and VCs is compensated by a reduction in total operating times, theatre utilisation, staffing and surgical waiting lists. Therefore, use of CTA and VCs has clear positive resource implications.
Although, there are multiple human and operational factors that may influence operative duration, the plastic surgery team used in all operations are experienced and complete on average two flaps per week. Thus, the results are unlikely to be affected by efficiency factors.
Conclusions
This is the first study which demonstrates the combined effect of both CTA and VCs in significantly reducing operative and ischaemia times for FFBR; the decrease being predominantly associated with use of CTA. We believe the reduction in operative time (by almost 2 hours) is because CTA, unlike VC use, facilitates surgery without a surgical learning curve and assists in surgical planning by providing a useful roadmap of the perforator vessels. Strong proponents of these two modalities would advocate their combined use for microvascular breast reconstruction as shorter operative times have clinical benefits for the patient. Surgical experience may also play a role in reducing surgery duration.
Acknowledgements
This work was supported by Mr Amer Durrani, Consultant Plastic Surgeon, who introduced the use of couplers to the Cambridge University Hospital in 2010.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was part of a quality improvement assessment and an ongoing departmental free flap audit. Audits and Quality Improvement Assessments are part of a larger framework for Clinical Governance, whereby National Health Service (NHS) organizations are accountable for continuously improving the quality of their services and safeguarding high standards. Clinical governance (inclusive of audits and quality improvement assessments) is compulsory within the NHS. No ethical committee approval is therefore needed. Under the Caldicott Principles, non-identifiable patient data can be collected, stored and used for purpose of audit without individual patient consent. Additionally, all data were collected retrospectively from hospital medical records. Therefore, individual patient consent was not required for this study. Subjects in clinical photographs gave informed consent for use of images in journal publication.
References
- Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg 1999;52:104-11. [Crossref] [PubMed]
- Nimalan N, Branford OA, Stocks G. Anaesthesia for free flap breast reconstruction British J Anaes Ed 2016;16:162-6.
- Pollhammer MS, Duscher D, Schmidt M, et al. Recent advances in microvascular autologous breast reconstruction after ablative tumor surgery. World J Clin Oncol 2016;7:114-21. [Crossref] [PubMed]
- Masia J, Kosutic D, Clavero JA, et al. Preoperative computed tomographic angiogram for deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg 2010;26:21-8. [Crossref] [PubMed]
- Alonso-Burgos A, Garcia-Tutor E, Bastarrika G, et al. Preoperative planning of deep inferior epigastric artery perforator flap reconstruction with multislice-CT angiography: imaging findings and initial experience. J Plast Reconstr Aesthet Surg 2006;59:585-93. [Crossref] [PubMed]
- Rozen WM, Garcia-Tutor E, Alonso-Burgos A, et al. R. Planning and optimising DIEP flaps with virtual surgery: the Navarra experience. J Plast Reconstr Aesthet Surg 2010;63:289-97. [Crossref] [PubMed]
- Neil-Dwyer JG, Ludman CN, Schaverien M, et al. Magnetic resonance angiography in preoperative planning of deep inferior epigastric artery perforator flaps. J Plast Reconstr Aesthet Surg 2009;62:1661-5. [Crossref] [PubMed]
- Rozen WM, Palmer KP, Suami H, et al. The DIEA branching pattern and its relationship to perforators: the importance of preoperative computed tomographic angiography for DIEA perforator flaps. Plast Reconstr Surg 2008;121:367-73. [Crossref] [PubMed]
- Ohkuma R, Mohan R, Baltodano PA, et al. Abdominally based free flap planning in breast reconstruction with computed tomographic angiography: systematic review and meta-analysis. Plast Reconstr Surg 2014;133:483-94. [Crossref] [PubMed]
- Vanschoonbeek A, Fabre G, Nanhekhan L, et al. Outcome after urgent microvascular revision of free DIEP, SIEA and SGAP flaps for autologous breast reconstruction J Plast Reconstr Aesthet Surg 2016;69:1598-608. [Crossref] [PubMed]
- Bui DT, Cordeiro PG, Hu QY, et al. Free flap reexploration: indications, treatment, and outcomes in 1193 free flaps. Plast Reconstr Surg 2007;119:2092-100. [Crossref] [PubMed]
- Gardiner MD, Nanchahal J. Strategies to ensure success of microvascular free tissue transfer. J Plast Reconstr Aesthet Surg 2010;63:e665-73. [Crossref] [PubMed]
- Kulkarni AR, Mehrara BJ, Pusic AL, et al. Venous thrombosis in handsewn versus coupled venous anastomoses in 857 consecutive breast free flaps. J Reconstr Microsurg 2016;32:178-82. [Crossref] [PubMed]
- Jandali S, Wu LC, Vega SJ, et al. 1000 consecutive venous anastomoses using the microvascular anastomotic coupler in breast reconstruction. Plast Reconstr Surg 2010;125:792-8. [Crossref] [PubMed]
- Fitzgerald O’Connor E, Rozen WM, Chowdhry M, et al. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland Surg 2016;5:88-92. [PubMed]
- Malhotra A, Chhaya N, Nsiah-Sarbeng P, et al. CT-guided deep inferior epigastric perforator (DIEP) flap localization --better for the patient, the surgeon, and the hospital. Clin Radiol 2013;68:131-8. [Crossref] [PubMed]
- Fitzgerald O’Connor E, Rozen WM, Chowdhry M, et al. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surg 2016;5:93-8. [PubMed]
- Grewal AS, Erovic B, Strumas N, et al. The utility of the microvascular anastomotic coupler in free tissue transfer. Can J Plast Surg 2012;20:98-102. [Crossref] [PubMed]
- Masia J, Clavero J, Larrañaga J, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-9. [Crossref] [PubMed]
- Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [Crossref] [PubMed]
- Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg 2009;62:1112-7. [Crossref] [PubMed]
- Casey WJ III, Chew RT, Rebecca AM, et al. Advantages of preoperative computed tomography in deep inferior epigastric artery perforator flap breast reconstruction. Plast Reconstr Surg 2009;123:1148-55. [Crossref] [PubMed]
- Minqiang X, Lanhua M, Jie L, et al. The value of multidetector-row CT angiography for pre-operative planning of breast reconstruction with deep inferior epigastric arterial perforator flaps. Br J Radiol 2010;83:40-3. [Crossref] [PubMed]
- Uppal RS, Casaer B, Van Landuyt K, et al. The efficacy of preoperative mapping of perforators in reducing operative times and complications in perforator flap breast reconstruction. J Plast Reconstr Aesthet Surg 2009;62:859-64. [Crossref] [PubMed]
- Ghattaura A, Henton J, Jallali N, et al. One hundred cases of abdominal-based free flaps in breast reconstruction. The impact of preoperative computed tomographic angiography. J Plast Reconstr Aesthet Surg 2010;63:1597-601. [Crossref] [PubMed]
- Rozen WM, Whitaker IS, Acosta R. Venous coupler for free-flap anastomosis: outcomes of 1,000 cases. Anticancer Res 2010;30:1293-4. [PubMed]
- Tong WM, Dixon R, Ekis H, et al. The impact of preoperative CT angiography on breast reconstruction with abdominal perforator flaps. Ann Plast Surg 2012;68:525-30. [Crossref] [PubMed]
- Vargas CR, Koolen PG, Ho OA, et al. Preoperative CT-angiography in autologous breast reconstruction. Microsurgery 2016;36:623-7. [Crossref] [PubMed]
- Malata CM, Moses M, Mickute Z, et al. Tips for successful microvascular abdominal flap breast reconstruction utilizing the “total rib preservation” technique for internal mammary vessel exposure. Ann Plast Surg 2011;66:36-42. [Crossref] [PubMed]
- Rozen WM, Garcia-Tutor E, Alonso-Burgos A, et al. The effect of anterior abdominal wall scars on the vascular anatomy of the abdominal wall: a cadaveric and clinical study with clinical implications. Clin Anat 2009;22:815-22. [Crossref] [PubMed]
- Hofer SO, Damen TH, Mureau MA, et al. A critical review of perioperative complications in 175 free deep inferior epigastric perforator flap breast reconstructions. Ann Plast Surg 2007;59:137-42. [Crossref] [PubMed]
- Busic V, Das-Gupta R, Mesic H, et al. The deep inferior epigastric perforator flap for breast reconstruction, the learning curve explored. J Plast Reconstr Aesthet Surg 2006;59:580-4. [Crossref] [PubMed]
- Rosich-Medina A, Bouloumpasis S, Di Candia M, et al. Total “rib”-preservation technique of internal mammary vessel exposure for free flap breast reconstruction: A 5-year prospective cohort study and instructional video. Ann Med Surg (Lond) 2015;4:293-300. [Crossref] [PubMed]
- Hardy KL, Davis KE, Constantine RS, et al. The impact of operative time on complications after plastic surgery: a multivariate regression analysis of 1753 cases. Aesthet Surg J 2014;34:614-22. [Crossref] [PubMed]


