
Strategies and considerations in selecting between subpectoral and prepectoral breast reconstruction
Introduction
Implant-based reconstruction remains the most common form of breast reconstruction in the United States today (1). Subpectoral implant reconstruction has been considered the standard of care in the 21st century, given concerns regarding complications associated with prepectoral implant placement. Since its original description in 1981 (2), techniques have been modified with the introduction of newer generation implants and expanders as well as the advancement of extirpative techniques from total to skin and nipple-preserving mastectomies (3,4). The advent of dual plane reconstruction using acellular dermal matrix (ADM) (5,6), and later mesh (7-9) further decreased the morbidity of total submuscular coverage requiring serratus muscle and/or fascia elevation. These advances also facilitated increased intraoperative tissue expander fill volumes along with improved lower pole aesthetics and the ability to offer single-stage immediate implant reconstruction (10).
Recently, prepectoral reconstruction has been revisited in a new light. Several advancements in both mastectomy and reconstructive techniques have allowed safe and efficacious subcutaneous implant placement. Refinements in skin-sparing and nipple-sparing mastectomy have maximized the amount of tissue that can be safely preserved while maintaining oncologic safety whereas the introduction of ADM and form-stable implants have minimized rates of capsular contracture and further facilitated the attainment of an aesthetic reconstruction by improving pocket control and implant positioning (11). Technologic advancements in perfusion monitoring, such as with indocyanine green angiography, have improved our ability to assess the viability of skin flaps and better select cases appropriate for subcutaneous implant placement. Fat grafting is an equally critical component of prepectoral reconstruction that increases the thickness of soft tissue flaps while minimizing the palpability and visibility of implants to enhance aesthetic outcomes.
As techniques and technology evolve, the number of reconstructive choices for patients and plastic surgeons continues to increase. Treatment must be individualized for each patient. Despite there often being more than one appropriate reconstructive option, choosing the best procedure requires a thorough understanding of the benefits and drawbacks of different techniques with regards to each specific patient and procedure. Subpectoral and prepectoral implant-based breast reconstruction can both provide excellent results; however, understanding the limitations and ideal applications of each technique will allow the surgeon to optimize outcomes and minimize complications.
Subpectoral breast reconstruction
Total submuscular and dual-plane breast reconstruction with ADM have both demonstrated low complication rates and proved successful reconstructive modalities when utilized appropriately (12). Total submuscular techniques, with implant placement under the pectoralis muscle and serratus muscle and/or fascia have traditionally been perceived to be the “safest” with regards to rates of postoperative complications such as seroma, infection and implant loss (13-16). Limited expansion of the inferior pole to mimic the natural curvature of the breast (17) as well as additional morbidity with serratus elevation led to the introduction of dual-plane procedures.
A variety of options for dual-plane implant placement currently exist, most commonly utilizing an adjunctive scaffold, such as ADM or mesh, to define the inframammary fold, provide inferolateral implant support and contour, as well as preventing window shading of the pectoralis muscle. The use of ADM and mesh has also allowed for larger pocket sizes to facilitate direct-to-implant reconstruction (10). Prospective studies have shown similar rates of complications among ADM-assisted and non-ADM implant reconstructions (18), though long-term capsular contracture rates with ADM-assisted reconstruction remain very low (19). Overall, more minimally invasive muscular dissections, advances in the technology of adjunctive materials and implants, and refinements in mastectomy techniques have yielded low complication rates and high patient satisfaction with the different variations of subpectoral implant reconstruction (12,20,21).
Prepectoral reconstruction
Prepectoral breast reconstruction techniques have recently become “re-popularized” as a less-invasive alternative to subpectoral breast reconstruction. Purported benefits include reduced pain and minimization of animation deformity. Compared to initial descriptions, critical technical advances, including refined ablative procedures, form stable prostheses, biologic and synthetic matrices/mesh, and adjunctive fat transfer procedures, have facilitated the re-introduction of subcutaneous implant-based breast reconstruction.
Initially, prepectoral breast reconstruction was reported in small series that employed both immediate implant and two-stage expander reconstructions using a variety of ADMs and meshes with low complications rates (22-26). These outcomes have been confirmed in larger multicenter trials (27). While surgical procedures vary, the majority of techniques utilize some form of ADM or mesh to control implant position, tailor the implant pocket and mitigate excessive pressure from implants on inferior mastectomy flaps. Most commonly this involves either an anterior sling (28-30) or a complete implant wrap (31-33). Prepectoral implant reconstruction without ADM or mesh has also been reported with low complications rates (34).
Comparative studies between prepectoral and subpectoral reconstruction have demonstrated similar acute and subacute complications rates (3,29,30,35-39). Importantly, rates of capsular contracture with ADM use have remained low (36). Preliminary results on cosmetic and patient-reported outcomes are promising (36,38), though further long-term studies are needed.
Preoperative considerations
Patient selection
Proper patient selection is absolutely critical for success in prepectoral reconstruction (40). As implants are placed beneath the skin and subcutaneous tissue, with no intervening vascularized muscle layer, all efforts must be made to ensure the predictability of mastectomy flap viability to avoid devastating implant exposure. While certain factors are inherently out of the surgeon’s control, maintaining strict exclusion criteria for prepectoral reconstruction will minimize the risk of having to alter the reconstructive plan intraoperatively. Relative contraindications include patients with comorbidities such as uncontrolled diabetes, morbid obesity, recent or current tobacco use, as well as those with a history of preoperative radiation (Table 1). These patients may benefit from implant placement either completely or partially under the pectoralis muscle to provide an additional vascularized layer between the reconstruction and potentially compromised skin flaps. Patients expected to receive postoperative radiation, on the other hand, may benefit from prepectoral implant placement as radiation-induced pectoralis fibrosis and distortion overlying the implant are avoided (41).

Full table
Breast size and ptosis are also important to consider. Increasing breast ptosis will result in a greater distance from the chest wall to the mastectomy flap edges, resulting in more compromised skin perfusion. In cases of prepectoral reconstruction, support of the implant with fixation of at least an anterior sheet of ADM/mesh to the chest wall will help to minimize pressure from the implant on mastectomy flaps. Skin reduction techniques, in both an immediate and delayed fashion, have been described in subpectoral (42) and prepectoral (43) reconstruction. These may be utilized in patients with large and/or ptotic breasts to reduce the skin envelope to better accommodate the underlying tissue expander or permanent implant.
The amount of subcutaneous tissue superficial to the breast capsule, or superficial breast fascia, varies among patients (44-46). Assessment of the subcutaneous breast layer is of utmost importance in determining ability to perform prepectoral implant placement as this is the sole tissue layer overlying the implant. Further, breast subcutaneous thickness will influence aesthetic outcomes, particularly with regards to contour deformities and skin rippling. Preoperative estimation of thickness with a pinch test will help assess whether subpectoral reconstruction may provide better upper pole contour than prepectoral implant placement (in patients within minimal subcutaneous tissue) or if ancillary procedures such as fat grafting and/or ADM placement may be needed to prevent/treat hollowing or rippling if prepectoral reconstruction is to be performed. Delayed reconstruction can also be considered in these patients. While these estimates are helpful, they are entirely dependent on the relative thickness of skin flaps after mastectomy and must therefore be re-evaluated intraoperatively. Importantly, thin patients are not contraindicated for prepectoral reconstruction (47); rather, the appropriate technical modifications, such as optimal preservation of the subcutaneous layer and adjunctive fat grafting, must be planned to optimize aesthetic results.
Oncologic considerations
As with any form of breast reconstruction, oncologic extirpation and postoperative monitoring for recurrent or new primary malignancies are of utmost importance. Very large tumors and gross axillary nodal involvement have been considered contraindications for implant placement (48). In the case of prepectoral reconstruction, additional consideration must be given to deep tumors or those along the chest wall (49). Imaging for postoperative screening can be limited in detecting chest wall recurrence, particularly with suprapectoral implants, and therefore subpectoral implants should be considered if oncologically feasible. The oncologic safety of prepectoral reconstruction is not fully elucidated in these cases while stage IV disease has been associated with increased expander complications in prepectoral reconstruction (37). Therefore, prepectoral reconstruction is generally contraindicated in cases of late cancer stage or any patients deemed at high risk of recurrence (50). In all cases, the plane of desired implant placement should be discussed with the oncologic surgeon preoperatively to formulate a mutual operative plan.
Reconstructive technique
Single-stage immediate implant and two-stage tissue expander reconstruction have both been reported with prepectoral breast reconstruction (30,36). Immediate implant reconstruction in itself has specific selection criteria including preoperative breast size and ptosis, skin quality and excess, patient co-morbidities, as well as patient preference for postoperative breast size (10). Notably, implant size greater than 400 mL has been associated with increased complications in immediate one-stage implant reconstruction (10). These factors are even more critical in prepectoral reconstruction as mastectomy flaps have less tolerance for an implant-pocket size mismatch resulting in increased tissue stress and risk of complications. Selection criteria is therefore more stringent. While no absolute contraindications exist, patients with multiple relative contraindications for one-stage implant reconstruction, such as age and smoking as enumerated elsewhere, should preferentially be offered two-stage reconstruction. Further, patients desiring a larger post-operative breast size or in whom immediate implant placement places too great stress on the mastectomy flaps are better served with two-stage reconstruction.
Patient preferences
An increasing number of bilateral prophylactic mastectomies (51) and immediate breast reconstructions (52) are being performed in younger patients at high risk for familial breast cancer. Young and active patients may prefer to avoid the morbidity of pectoralis disinsertion. At the severe end of this spectrum is animation deformity, which can be a nuisance to patients in milder cases or cause significant pain and aesthetic deformity in severe cases (53,54). Even subtle animation deformity can be particularly bothersome for athletes or women that regularly perform upper body exercise. In these patients, prepectoral reconstruction may be particularly beneficial to overall quality of life (55).
Shared decision-making is a critical aspect of the preoperative consultation (56,57). Patients must not only be aware, but thoroughly understand, the implications of each reconstructive technique. For example, potentially higher rates of rippling and need for revision, including fat grafting, in prepectoral reconstructions must be weighed against the potential animation deformity in subpectoral reconstruction if no clear technique preference is evident. This process of shared-decision making will allow patient and plastic surgeon to mutually decide on treatment plans and arrive at realistic expectations for surgical outcomes. Importantly, patients must understand that the final decision to pursue prepectoral or subpectoral reconstruction occurs intraoperatively, and can change based on certain intraoperative factors to provide the best and safest reconstruction for the patient.
Intraoperative considerations
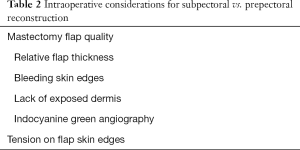
The most critical aspect in success of prepectoral reconstruction is the quality of mastectomy flaps (Table 2). While mastectomy flap necrosis is a potentially devastating complication regardless off reconstructive technique, it represents an even greater reconstructive threat with prepectoral implant placement as no vascularized tissue is interposed between the compromised skin and underlying implant. Well-perfused mastectomy skin and a nipple-areola complex (in nipple-sparing mastectomy) flaps are mandatory in any case of prepectoral reconstruction. This requires maintaining the superficial circulation of the breast by preserving the subcutaneous breast layer by precise dissection at the level of the superficial breast fascia.
Full table
After evaluation of patient candidacy for prepectoral implant placement on an individual basis, the next key decision point in choosing subpectoral vs. prepectoral implant placement is during the evaluation of mastectomy flaps intraoperatively. Ideally, an operative plan is formulated with the breast surgeon after evaluating preoperative imaging for the thickness of subcutaneous tissue anterior to the breast capsule, the location of the tumor and areas of extension of breast tissue to the superficial hypodermis. Magnetic resonance imaging (MRI) evaluation of pre- and post-operative breasts have shown that though the thickness of the subcutaneous layer is highly variable among patients, a significantly increased rate of ischemic complications is seen with a lower ratio of overall postoperative to preoperative flap thickness and postoperative flap thickness below an absolute thickness of 8.0 mm (46).
Preemptive evaluation and a coordinated discussion of these factors with the oncologic surgeon can help avoid unwanted surprises in the operating room after completion of mastectomy. At this time, mastectomy flaps must be thoroughly assessed for quality and viability. Clinically, this entails examining the flaps for signs of adequate perfusion such as bleeding at skin edges, preservation of subcutaneous fat under skin flaps and lack of exposed dermis (40). It is important to note that while absolute thickness is not always predictive of perfusion, preserved relative thickness to patient’s preoperative subcutaneous layer is an important surrogate for perfusion (46). Indocyanine green angiography is also a useful adjunctive tool to assess for tissue perfusion if epinephrine-containing solutions have not been utilized. Any concern for poor mastectomy flap quality represents an indication for subpectoral implant placement to allow for an additional vascularized layer of tissue between the implant and compromised skin flaps. In cases of questionable mastectomy flap viability in patients who will not tolerate subpectoral implant placement, reconstruction may be deferred to delay the skin flaps prior to prepectoral device placement in a delayed fashion.
Other important factors at the time of surgery include tension on skin flaps if large amounts of skin have been resected. Excess tension can also predispose to ischemia at flap edges in which case immediate prepectoral reconstruction should be avoided. Furthermore, intraoperative evaluation of flap thickness permits assessment of the potential need for adjunctive techniques to optimize aesthetic results. Thin skin flaps with a paucity of subcutaneous tissue can lead to implant visibility, palpability and ripping. In such cases, a subpectoral reconstruction may provide additional soft tissue support to minimize these complications. On the other hand, these issues can be treated with ADM placement and/or immediate or delayed fat grafting.
Postoperative considerations
Goals of implant-based reconstruction regardless of implant location in the sub- or pre-pectoral plane include obtaining an aesthetic reconstruction with minimal complications in the acute and long-term postoperative period. Each reconstructive technique possesses unique considerations with a predisposition to particular long-term complications that require close monitoring and diagnosis. In certain cases, these complications may warrant implant pocket change from subpectoral to prepectoral, or vice versa. This underscores the importance of plastic surgeons being facile with both prepectoral and subpectoral techniques.
Subpectoral to prepectoral
Subpectoral to prepectoral pocket change is typically performed for animation deformity. The prevalence of animation deformity has been found to be higher in breast reconstruction compared to breast augmentation, with a minority, but still significant portion, reporting severe symptoms (54). Pocket change to the prepectoral space typically involves detachment and reinsertion of the pectoralis to the chest wall (with or without anterior capsulectomy), creation of a neo-pocket in the subcutaneous plane, and prepectoral implant placement with ADM/mesh reinforcement similar to primary reconstruction cases. Series on prepectoral revisions have reported resolution of animation deformity in the vast majority of cases with low complication rates (58,59).
Prepectoral to subpectoral
Implant exchange from the prepectoral to subpectoral space has traditionally been performed for severe cases of capsular contracture, in addition to total capsulectomy, when implants were originally placed in the subcutaneous plane. Reported rates of capsular contracture in prepectoral reconstruction with ADM have been low (36), and therefore the incidence of need for subpectoral pocket change may be decreasing. Long-term outcomes with regards to capsular contracture with prepectoral reconstruction, however, remains to be fully elucidated and limits the conclusions that can be made.
Pocket change from the prepectoral to subpectoral plane can also be performed to treat sequelae of soft tissue deficiency over the implant such as rippling and implant visibility or palpability (Table 3). Transition to the subpectoral plane can augment soft tissue thickness overlying the implant and can be utilized in combination with other revisional techniques such as ADM placement and fat grafting. Often the latter procedures may suffice for treating small deformities; however, implant exchange to the subpectoral plane can aid with more severe cases, particularly when the breast skin flap is very thin.

Full table
Conclusions
Prepectoral breast reconstruction has the potential to minimize the morbidity associated with submuscular reconstruction and animation deformity with promising reconstructive outcomes. The success of prepectoral reconstruction, however, is highly reliant on preoperative patient selection and intraoperative decision-making. While some patients will have excellent outcomes with prepectoral implant placement, others will be better served by either dual-plane or total subpectoral placement. The factors that influence this decision include various preoperative patient characteristics and intraoperative markers of mastectomy flap quality. Understanding the benefits and limitations of each technique will help the plastic surgeon decide on the appropriate procedure that will optimize reconstructive results and patient satisfaction while minimizing complications. Importantly, these decisions must be made together with the patient and ablative surgeon in order to arrive at the best oncologic and reconstructive outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Society of Plastic Surgeons. 2017 Plastic Surgery Statistics Report. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-report-2017.pdf
- Apfelberg DB, Laub DR, Maser MR, et al. Submuscular breast reconstruction--indications and techniques. Ann Plast Surg 1981;7:213-21. [Crossref] [PubMed]
- Bailey CR, Ogbuagu O, Baltodano PA, et al. Quality-of-Life Outcomes Improve with Nipple-Sparing Mastectomy and Breast Reconstruction. Plast Reconstr Surg 2017;140:219-26. [Crossref] [PubMed]
- Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol 2008;34:143-8. [Crossref] [PubMed]
- Breuing KH, Colwell AS. Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg 2007;59:250-5. [Crossref] [PubMed]
- Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9. [Crossref] [PubMed]
- Baldelli I, Cardoni G, Franchelli S, et al. Implant-Based Breast Reconstruction Using a Polyester Mesh (Surgimesh-PET): A Retrospective Single-Center Study. Plast Reconstr Surg 2016;137:931e-9e. [Crossref] [PubMed]
- Meyer Ganz O, Tobalem M, Perneger T, et al. Risks and benefits of using an absorbable mesh in one-stage immediate breast reconstruction: a comparative study. Plast Reconstr Surg 2015;135:498e-507e. [Crossref] [PubMed]
- Haynes DF, Kreithen JC. Vicryl mesh in expander/implant breast reconstruction: long-term follow-up in 38 patients. Plast Reconstr Surg 2014;134:892-9. [Crossref] [PubMed]
- Choi M, Frey JD, Alperovich M, et al. "Breast in a Day": Examining Single-Stage Immediate, Permanent Implant Reconstruction in Nipple-Sparing Mastectomy. Plast Reconstr Surg 2016;138:184e-91e. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. Bioengineered Breast: Concept, Technique, and Preliminary Results. Plast Reconstr Surg 2016;137:415-21. [Crossref] [PubMed]
- Weichman KE, Wilson SC, Saadeh PB, et al. Sterile "ready-to-use" AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg 2013;132:725-36. [Crossref] [PubMed]
- Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:1049-58. [Crossref] [PubMed]
- Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg 2010;125:1606-14. [Crossref] [PubMed]
- Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 2010;125:429-36. [Crossref] [PubMed]
- Liu AS, Kao HK, Reish RG, et al. Postoperative complications in prosthesis-based breast reconstruction using acellular dermal matrix. Plast Reconstr Surg 2011;127:1755-62. [Crossref] [PubMed]
- Glasberg SB, Light D. AlloDerm and Strattice in breast reconstruction: a comparison and techniques for optimizing outcomes. Plast Reconstr Surg 2012;129:1223-33. [Crossref] [PubMed]
- Sorkin M, Qi J, Kim HM, et al. Acellular Dermal Matrix in Immediate Expander/Implant Breast Reconstruction: A Multicenter Assessment of Risks and Benefits. Plast Reconstr Surg 2017;140:1091-100. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Berry C, et al. Acellular Dermal Matrix-Assisted Direct-to-Implant Breast Reconstruction and Capsular Contracture: A 13-Year Experience. Plast Reconstr Surg 2016;138:329-37. [Crossref] [PubMed]
- Susarla SM, Ganske I, Helliwell L, et al. Comparison of clinical outcomes and patient satisfaction in immediate single-stage versus two-stage implant-based breast reconstruction. Plast Reconstr Surg 2015;135:1e-8e. [Crossref] [PubMed]
- Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg 2014;133:496-506. [Crossref] [PubMed]
- Casella D, Bernini M, Bencini L, et al. TiLoop(R) Bra mesh used for immediate breast reconstruction: comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series. Eur J Plast Surg 2014;37:599-604. [Crossref] [PubMed]
- Casella D, Calabrese C, Bianchi S, et al. Subcutaneous Tissue Expander Placement with Synthetic Titanium-Coated Mesh in Breast Reconstruction: Long-term Results. Plast Reconstr Surg Glob Open 2016;3:e577. [Crossref] [PubMed]
- Becker H, Lind JG 2nd, Hopkins EG. Immediate Implant-based Prepectoral Breast Reconstruction Using a Vertical Incision. Plast Reconstr Surg Glob Open 2015;3:e412. [Crossref] [PubMed]
- Berna G, Cawthorn SJ, Papaccio G, et al. Evaluation of a novel breast reconstruction technique using the Braxon acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg 2017;87:493-8. [Crossref] [PubMed]
- Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg 2015;68:162-7. [Crossref] [PubMed]
- Vidya R, Masia J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: First multicenter European report on 100 cases. Breast J 2017;23:670-6. [Crossref] [PubMed]
- Jones G, Yoo A, King V, et al. Prepectoral Immediate Direct-to-Implant Breast Reconstruction with Anterior AlloDerm Coverage. Plast Reconstr Surg 2017;140:31S-8S. [Crossref] [PubMed]
- Nahabedian MY, Cocilovo C. Two-Stage Prosthetic Breast Reconstruction: A Comparison Between Prepectoral and Partial Subpectoral Techniques. Plast Reconstr Surg 2017;140:22S-30S. [Crossref] [PubMed]
- Sbitany H, Piper M, Lentz R. Prepectoral Breast Reconstruction: A Safe Alternative to Submuscular Prosthetic Reconstruction following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;140:432-43. [Crossref] [PubMed]
- Highton L, Johnson R, Kirwan C, et al. Prepectoral Implant-Based Breast Reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1488. [Crossref] [PubMed]
- Jafferbhoy S, Chandarana M, Houlihan M, et al. Early multicentre experience of pre-pectoral implant based immediate breast reconstruction using Braxon((R)). Gland Surg 2017;6:682-8. [Crossref] [PubMed]
- Vidya R. Prepectoral Breast Reconstruction or Muscle-Sparing Technique with the Braxon Porcine Acellular Dermal Matrix. Plast Reconstr Surg Glob Open 2017;5:e1364. [Crossref] [PubMed]
- Salibian AH, Harness JK, Mowlds DS. Staged Suprapectoral Expander/Implant Reconstruction without Acellular Dermal Matrix following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;139:30-9. [Crossref] [PubMed]
- Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg 2016;69:e77-86. [Crossref] [PubMed]
- Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous Direct-to-Implant Breast Reconstruction: Surgical, Functional, and Aesthetic Results after Long-Term Follow-Up. Plast Reconstr Surg Glob Open 2016;3:e574. [Crossref] [PubMed]
- Bettinger LN, Waters LM, Reese SW, et al. Comparative Study of Prepectoral and Subpectoral Expander-Based Breast Reconstruction and Clavien IIIb Score Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1433. [Crossref] [PubMed]
- Baker BG, Irri R, MacCallum V, et al. A Prospective Comparison of Short-Term Outcomes of Subpectoral and Prepectoral Strattice-Based Immediate Breast Reconstruction. Plast Reconstr Surg 2018;141:1077-84. [Crossref] [PubMed]
- Chatterjee A, Nahabedian MY, Gabriel A, et al. Early assessment of post-surgical outcomes with pre-pectoral breast reconstruction: A literature review and meta-analysis. J Surg Oncol 2018;117:1119-30. [Crossref] [PubMed]
- Sbitany H. Important Considerations for Performing Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:7S-13S. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction and Postmastectomy Radiotherapy: Short-Term Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1631. [Crossref] [PubMed]
- Derderian CA, Karp NS, Choi M. Wise-pattern breast reconstruction: modification using AlloDerm and a vascularized dermal-subcutaneous pedicle. Ann Plast Surg 2009;62:528-32. [Crossref] [PubMed]
- Caputo GG, Marchetti A, Dalla Pozza E, et al. Skin-Reduction Breast Reconstructions with Prepectoral Implant. Plast Reconstr Surg 2016;137:1702-5. [Crossref] [PubMed]
- Larson DL, Basir Z, Bruce T. Is oncologic safety compatible with a predictably viable mastectomy skin flap? Plast Reconstr Surg 2011;127:27-33. [Crossref] [PubMed]
- Beer GM, Varga Z, Budi S, et al. Incidence of the superficial fascia and its relevance in skin-sparing mastectomy. Cancer 2002;94:1619-25. [Crossref] [PubMed]
- Frey JD, Salibian AA, Choi M, et al. Mastectomy Flap Thickness and Complications in Nipple-Sparing Mastectomy: Objective Evaluation using Magnetic Resonance Imaging. Plast Reconstr Surg Glob Open 2017;5:e1439. [Crossref] [PubMed]
- Gabriel A, Maxwell GP. Prepectoral Breast Reconstruction in Challenging Patients. Plast Reconstr Surg 2017;140:14S-21S. [Crossref] [PubMed]
- Maxwell GP, Storm-Dickerson T, Whitworth P, et al. Advances in nipple-sparing mastectomy: oncological safety and incision selection. Aesthet Surg J 2011;31:310-9. [Crossref] [PubMed]
- Storm-Dickerson T, Sigalove N. Prepectoral Breast Reconstruction: The Breast Surgeon's Perspective. Plast Reconstr Surg 2017;140:43S-8S. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Cemal Y, Albornoz CR, Disa JJ, et al. A paradigm shift in U.S. breast reconstruction: Part 2. The influence of changing mastectomy patterns on reconstructive rate and method. Plast Reconstr Surg 2013;131:320e-6e. [Crossref] [PubMed]
- Fitzpatrick AM, Gao LL, Smith BL, et al. Cost and outcome analysis of breast reconstruction paradigm shift. Ann Plast Surg 2014;73:141-9. [Crossref] [PubMed]
- Spear SL, Schwartz J, Dayan JH, et al. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg 2009;33:44-8. [Crossref] [PubMed]
- Nigro LC, Blanchet NP. Animation Deformity in Postmastectomy Implant-Based Reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1407. [Crossref] [PubMed]
- Vidya R, Iqbal FM. A Guide to Prepectoral Breast Reconstruction: A New Dimension to Implant-based Breast Reconstruction. Clin Breast Cancer 2017;17:266-71. [Crossref] [PubMed]
- Lee CN, Deal AM, Huh R, et al. Quality of Patient Decisions About Breast Reconstruction After Mastectomy. JAMA Surg 2017;152:741-8. [Crossref] [PubMed]
- Lee CN, Ubel PA, Deal AM, et al. How Informed Is the Decision About Breast Reconstruction After Mastectomy?: A Prospective, Cross-sectional Study. Ann Surg 2016;264:1103-9. [Crossref] [PubMed]
- Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral Revision Breast Reconstruction for Treatment of Implant-Associated Animation Deformity: A Review of 102 Reconstructions. Aesthet Surg J 2018;38:519-26. [Crossref] [PubMed]
- Hammond DC, Schmitt WP, O'Connor EA. Treatment of breast animation deformity in implant-based reconstruction with pocket change to the subcutaneous position. Plast Reconstr Surg 2015;135:1540-4. [Crossref] [PubMed]


