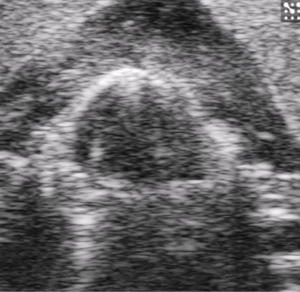
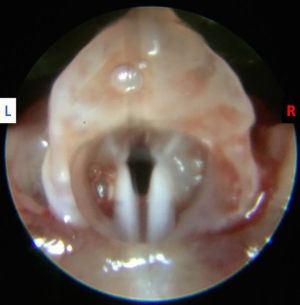
Validation of ultrasound as a diagnostic tool to assess vocal cord motion in an animal feasibility study
Introduction
Post thyroidectomy dysphonia can result from recurrent laryngeal nerve (RLN) injury. Because postoperative vocal cord paresis or palsy is not only an important procedure related complication, but also a major contributor to medicolegal litigation in thyroid surgery, information on the patient’s preoperative and postoperative vocal cord status is considered essential. However, the actual incidence of preoperative vocal cord paresis is relatively rare, and a great majority of patients would be subjected to unnecessary, uncomfortable laryngeal examination. Surgeon-performed transcutaneous laryngeal ultrasonography has been shown recently to be a promising, noninvasive tool in vocal cord examination (1). Apart from its non-invasiveness, it adds very little extra cost, because it can be performed as part of the preoperative examination of the thyroid gland and its regional lymph nodes and it takes as little as a few minutes (2). In the preoperative setting, the identification of vocal cord paresis may help the surgeon to better plan the operation. Postoperative laryngeal ultrasound helps the operating surgeon to analyze his or her nerve-injury related complication rate so as to avoid or decrease future occurrence.
Confirmation of postoperative recurrent nerve function has prompted many surgeons to advocate laryngoscopic examination. Indirect and flexible laryngoscopy permit visualization of vocal fold motion, however not all thyroid surgeons are skilled in these techniques. Both indirect and flexible fiberoptic examination can be uncomfortable for the patient. Indirect laryngoscopy, as performed with a mirror and a headlamp, has a significant failure rate due to gag reflexes and obstructing anatomy. Flexible fiberoptic laryngoscopy is the current gold standard for diagnosis of vocal fold paresis. However perioperative examination is not always possible due to lack of equipment or training. Direct laryngoscopy causes patient discomfort, requires special instrumentation and/or expertise, and is more expensive than ultrasonography (3). Recent studies suggest the vocal fold ultrasound as an alternative to flexible laryngoscopy. It offers the advantage of being non-invasive, and painless without radiation exposure or sedation. Whereas ultrasound has been compared to laryngoscopy in the clinical setting, there remains a need for correlation of laryngeal ultrasound results with known neurophysiology in the normal and injured state. Cheng et al. in 2012 reported that surgeon-performed ultrasound appears to be a relatively accurate method for assessing vocal cord movement in the perioperative setting (4). It can be used to select patients to undergo laryngoscopic examination before thyroidectomy and parathyroidectomy. The accessibility rates in the preoperative and postoperative settings were 96% and 95% respectively as reported by Wang et al. in their 2012 study of surgeon-performed transcutaneous laryngeal ultrasound (5). In the era of minimally invasive procedures and cost containment, the implication of this is great, because potentially fewer patients will require direct laryngoscopy before and after thyroidectomy and would be subjected to an unnecessary procedure.
The current gold standard for evaluation of vocal cord palsy is the indirect flexible fiberoptic laryngoscopy. Because true vocal cord (TVC) paralysis is not always apparent clinically, especially when the abnormal TVC is medialized, some surgeons recommend indirect flexible laryngoscopy for all patients before and after a central neck surgery (thyroidectomy, parathyroidectomy, anterior approach to cervical spine). However, performing indirect flexible laryngoscopy on all patients may not be cost effective. Equipment and training are not always available. Indirect flexible laryngoscopy is invasive, may require specialized instrumentation, can be unpleasant for patient, and may require an additional appointment with another specialist.
We aim to validate the use of ultrasonography in the diagnosis of TVC paresis. Over the past decades, TVC ultrasonography has been shown to be accurate in determining TVC mobility with a high sensitivity compared to flexible laryngoscopy. Because cervical surgeon-performed ultrasound has become part of the surgical evaluation for many surgeons specialized in endocrine surgery, this method of evaluating TVC mobility could become a real asset in the preoperative and postoperative management of patients undergoing thyroid and parathyroid operations. This ultrasound procedure is noninvasive and is not associated with either pain or injury and is used widely for tumor evaluations in small animals. We seek to shift current clinical practice paradigms with this validation of ultrasonography as a diagnostic method of postoperative TVC paresis. This offers an attractive alternative to flexible laryngoscopy that is non-invasive, and painless without radiation exposure or sedation.
A rat model of the RLN has been established, in which rigid endoscopy and transoral laryngeal electromyography (EMG) can be employed serially to follow a standardized RLN injury (6). This model has been used to study the histologic and electromyographic evolution of a standardized nerve crush injury modeling neuropraxia and axonotmesis. We wished to use this model to establish a correlation between the neurophysiology of both the injured and normal RLN with the direct endoscopic findings and with the laryngeal ultrasound findings. We attempted to evaluate both biologic (neurophysiology) and functional (cord movement) features of interest. The correlation of the biologic and functional aspects of RLN injury will further improve the applicability of this animal study to the characterization, prevention, and treatment of RLN injury.
The rat has been used extensively to create a model of acquired vocal cord paralysis in studies investigating various methods of RLN repair and recovery. Its anatomy is ideal for accessing the RLN for both injury and repair, and the laryngeal anatomy has proven to be readily evaluated via endoscopic visualization. Ultrasound evaluation of the anatomy and function of the larynx has been used in veterinary medicine in the canine, feline, and equine species. However, there is no added advantage to studying larger species than the rat, and the costs, anesthesia and maintenance complexities do not support a species other than the rat. The larynx is a dynamic, complex, three-dimensional system, and validating a diagnostic technique aimed at baseline and post-injury characteristics cannot be replicated in an in vitro, computer, static or other non-animal model.
There were three aims of our study. The first aim was to validate the use of ultrasound as a clinical tool in the diagnosis of true vocal fold palsy. We wanted to calculate the sensitivity and specificity of ultrasound for the diagnosis of true vocal fold palsy. We aimed to elucidate inter-rater consistency and estimate ease of recognition of ultrasound patterns associated with mobile or immobile vocal cords. The translational value of this study lies in its validation of clinical use of ultrasound in human vocal cord dysfunction as a broadly applicable diagnostic technique. Our second aim was to correlate laryngeal ultrasound findings with known neurophysiology in the normal and injured state in the normal and injured state of the rat RLN as well as with a standard diagnostic method (laryngeal endoscopy). Our third aim was to establish the feasibility of the use of the rat model for the study of ultrasound of the larynx.
Methods
Approval for this study was obtained from the Institutional Animal Care & Use Committee at the University of Tennessee (IACUC ID 15-002.0-A). After a 7-day acclimation period, four Sprague Dawley male rats underwent central neck surgery. General anesthesia with spontaneous respiration was performed. Intraperitoneal ketamine (50 mg/kg) and xylazine (5 mg/kg) were administered after inhalation of 1.8% isoflurane in a vented chamber was used to induce anesthesia. A nose cone allowing repeated exposure to inhalational agent was fitted to prevent premature emergence from anesthesia. Each rat was placed supine on the operating surface fitted for use with the Vevo 2100 high-frequency ultrasound imaging equipment, which was also fitted to record electrocardiography/monitor heart rate. A small amount of electrode gel was placed on the animals four feet and they were secured to the platform with tape. A rectal temperature probe was inserted after lubricating with gel.
Throughout the procedure, anesthetic depth, heart rate, body temperature, ECG, and respiratory rate were monitored/recorded using the Visual Sonics Vevo 2100 system. Each animal maintained a body temperature of 37.5 °C (±0.5 °C), heart rate 450 bpm (±50 bpm), and a normal QRST wave.
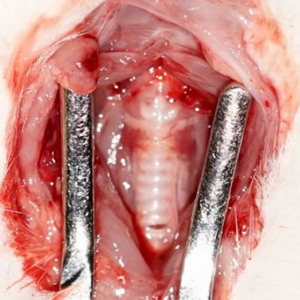
The tongue was retracted with a 3.0 silk suture placed in the midline in the anterior one third of the tongue and suspended. A 30-degree Storz 2.5 mm rigid endoscope equipped was transorally inserted to visualize the endolarynx and provide excellent visualization of the glottal region (Figures 1 and 2).


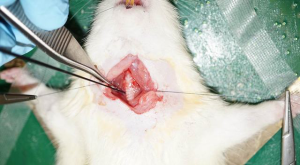
After the laryngeal endoscope was stabilized, the anterior neck skin was incised through the midline to expose the strap muscles which were retracted laterally, exposing the larynx, trachea, thyroid gland, and RLNs, using loupes for magnification (Figures 3-5). EMG needle electrodes from the MedTronic Neural Integrity Monitor (NIM) were inserted into bilateral thyroarytenoid muscles, per the protocol described by Tessema (6).
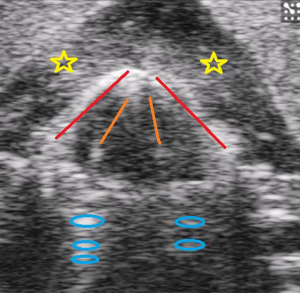
Ultrasound gel, which was centrifuged for clarity, was placed within the operative field and a 40 mHz linear array transducer positioned in contact with the gel, but at a height to optimize imaging of the endolarynx while still allowing access to the RLNs. Ultrasound gel was placed over the area of study and image acquisition started. Several 2D and 3D images were recorded. The images were stored, so that various measurements could be done at a later time, thus allowing for a shorter anesthetic event (~30 minutes).
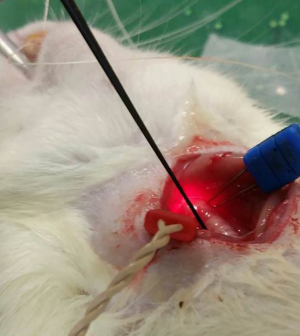
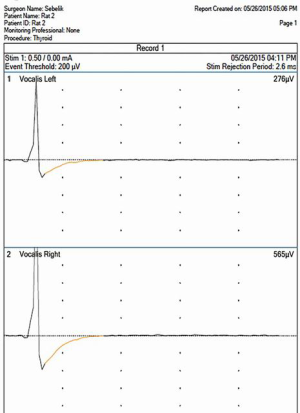
Two hand-held battery-powered nerve stimulators set at 0.2 mAmps were placed against the RLNs to provide simultaneous bilateral nerve stimulation (Figures 6 and 7). Evidence of vocal cord motion was then recorded via ultrasound image video (Figures 8 and 9) clips, endoscopic video clips (Figures 10 and 11), and action potentials on the NIM.
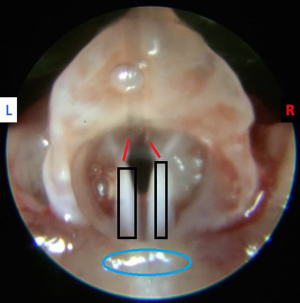
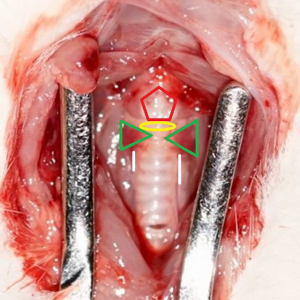
Once the pre-injury vocal cord motion was stimulated and recorded, one of the RLNs was injured by resecting a 5 mm segment of the nerve proximal to the larynx in the paratracheal region. The contralateral RLN was not damaged, and it served as a control. From the group of 4 rats, 2 underwent injury of the right RLN, and 2 underwent injury of the left RLN. Following injury of the RLN, both nerves were stimulated (the injured nerve stimulated proximal to the injury) and vocal cord motion recorded via the 3 methods as described above (ultrasound, endoscopy, and neural monitoring). Recordings of ultrasound and endolaryngeal examinations were indexed to the “gold standard” of vocal cord function, the neuromuscular action potential.
This was a terminal procedure for the rats. Following recording of neuromuscular action potentials, ultrasound, and endoscopic examinations of both the injured side and normal side of the larynx the rats were administered a lethal dose of anesthetic. Demise was confirmed by cessation of physiologic activity. At the conclusion of the procedure the rat was removed from the platform and wiped dry with gauze.
The results of both imaging modalities were compared to the presence of neuromuscular action potentials following stimulation of the RLN. The sensitivity and specificity of the ultrasound and endoscopy was calculated and the rate of agreement between the two imaging modalities was calculated.
Results
All 4 animals yielded readily visible vocal cord motion. We noted that higher frequency of ultrasound provided better detail and adequate depth. The 40 mHz had better quality than 30 mHz in the rat subject. The frequency in current human use for the larynx is ~10–12 mHz. We were able to surgically access the recurrent nerves and place extra-laryngeal monitor needles while the ultrasound probe was in place, although this was found to be challenging. The investigators observed a 100% correlation between endoscopic and ultrasonographic assessments.
Discussion
The translational value of this study lies in its validation of clinical use of ultrasound in human vocal cord dysfunction as a broadly applicable diagnostic technique. We were successful at validating the use of ultrasound as a diagnostic clinical tool in the diagnosis of true vocal fold palsy in the rat. We showed the sensitivity and specificity of ultrasound for the diagnosis of true vocal fold palsy to be 100%. Laryngeal ultrasound findings correlated with known neurophysiology in the normal and injured state in the normal and injured state of the rat RLN as well as with a standard diagnostic method (laryngeal endoscopy). Laryngoscopy and ultrasound agreed 100% of the time in determining vocal cord paralysis. We confirmed the reliability of ultrasound as a diagnostic tool to assess vocal cord motion. Ultrasound produced measurable images in the rat model in 100% of our subjects. The rat was found to be a very feasible model for the study of ultrasound of the larynx.
There were limitations to our study. The ability to access muscles supplied by the RLN via extra-laryngeal approach was limited by ultrasound probe placement. Developing a “NIM” tube analog to enhance vocal cord neuromuscular observations similar to the one used in human surgery would be helpful. The ability to access RLNs for stimulation before and after injury was also limited by ultrasound probe placement. Changing to continuous vagus nerve monitoring (placement of connector to the vagus nerve within the carotid sheath) to allow continuous monitoring and stimulation remote from central neck manipulation would be helpful.
Our next stage in the study includes five blinded professionals to identify right, left, or bilateral vocal cord motion on ultrasound clips for inter-rater reliability. Five physicians, each with varying degrees of expertise in vocal cord dysfunction, blinded to the presence or absence of neuromuscular activity, will be asked to rate the video clips of ultrasound and endoscopic imaging and to state whether bilateral vocal cords are mobile, right vocal cord is mobile, left cord is mobile, or neither cord is mobile. This will help support using the ultrasound as a research tool to follow RLN injury and recovery in the animal model. Our results will justify wider study and use of vocal cord ultrasound in the clinical setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval for this study was obtained from the Institutional Animal Care & Use Committee at the University of Tennessee (IACUC ID 15-002.0-A).
References
- Chandrasekhar SS, Randolph GW, Seidman MD, et al. Clinical practice guideline: improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg 2013;148:S1-37. [Crossref] [PubMed]
- Shaha AR. Routine laryngoscopy in thyroid surgery: A valuable adjunct. Surgery 2007;142:865-6. [Crossref] [PubMed]
- Schlosser K, Zeuner M, Wagner M, et al. Laryngoscopy in thyroid surgery--essential standard or unnecessary routine? Surgery 2007;142:858-64; discussion 864.e1-2.
- Cheng SP, Lee JJ, Liu TP, et al. Preoperative ultrasonography assessment of vocal cord movement during thyroid and parathyroid surgery. World J Surg 2012;36:2509-15. [Crossref] [PubMed]
- Wang CP, Chen TC, Yang TL, et al. Transcutaneous ultrasound for evaluation of vocal fold movement in patients with thyroid disease. Eur J Radiol 2012;81:e288-91. [Crossref] [PubMed]
- Tessema B, Roark RM, Pitman MJ, et al. Observations of recurrent laryngeal nerve injury and recovery using a rat model. Laryngoscope 2009;119:1644-51. [Crossref] [PubMed]