Osteoporosis and musculoskeletal complications related to therapy of breast cancer
Introduction
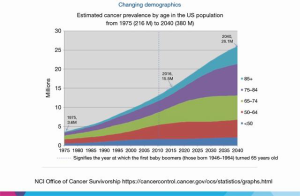
The majority of women diagnosed today with early-stage breast cancer will be long-term survivors and experience personal cures (1). Hence, awareness, screening, and treating the late and long-term effects of breast cancer treatments are of paramount importance in maintaining health (2). One of the common long-term effects of breast cancer treatments is osteoporosis. Chemotherapy-related ovarian failure, gonadotropin hormone-releasing (GnRH) agonists, and aromatase inhibitors (AIs) all result in bone loss that in some women is of sufficient magnitude to cause osteoporosis and fractures. The risk of osteoporosis is higher in postmenopausal women with breast cancer receiving AIs (3). Osteoporosis is highly relevant to the 25 million cancer survivors in the United States (US) by the year 2040, the majority of whom will be in their sixth, seventh, and eighth decades (Figure 1).
Thus, as both breast cancer and osteoporosis increase with age, screening, prevention, and treatment of osteoporosis in women with breast cancer are high priorities. Postmenopausal women with estrogen receptor (ER)-positive breast cancers (i.e., luminal A and B subtypes (4) representing about seventy-five of all breast cancers. Currently, AIs are an important part of the standard treatment for early stage and advanced ER-positive breast cancers. The focus of this review is on AI-related osteoporosis (5,6), arthralgia, and myalgia (7-9).
Aromatase
Aromatase (CYP19) is in the family cytochrome P450 enzymes that are responsible for converting adrenal gland-derived androgens to estrogens in postmenopausal women (10). Many tissues including ovarian, adipose, bone, healthy breast, breast cancer, and brain contain CYP19. Inhibiting aromatase results in decreasing estrogen levels lower than the already low estrogen levels of postmenopausal women (11). Anastrozole, exemestane, and letrozole are the three selective AIs in use for the treatment of postmenopausal women with breast cancers expressing the estrogen (ER) and/or progesterone receptor (PR). Randomized trials and meta-analysis show the superiority of AIs over tamoxifen in disease-free and overall survival (12,13).
All three AIs have similar benefits (14); they reduce the risks of local, distant recurrence, contralateral breast cancers, and improve overall survival in comparison to tamoxifen. They also have the same spectrum of side effects including possible increases in vasomotor symptoms, arthralgias and myalgias, vaginal dryness, hair thinning, and bone loss leading higher risks of osteoporosis and fractures.
Aromatase and bone loss: mechanisms of action
Aromatase regulates estrogen in postmenopausal women (15), and estrogens and other hormones play a central role in the regulation of bone mass (16). The control of healthy bone mass involves two levels: at a “macro” level, the regulation of healthy bone is through systemic hormones (e.g., androgens, estrogens, calcitonin, and parathyroid hormone), and the mechanical forces imposed by gravity. The “micro” level occurs in the bone-remodeling unit, comprised of two primary cell types. Osteoblasts, derived from mesenchymal precursor cells, are responsible for new bone formation, whereas, osteoclasts derived from hematopoietic precursor cells are responsible for bone resorption.
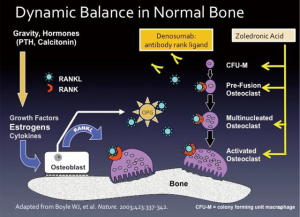
The dynamic balance between osteoblast and osteoclast function regulates new bone formation and resorption (Figure 2) (17). The master regulator is the osteoblast secreting both osteoprotegerin (OPG; also called osteoclastogenesis inhibitory factor, a member of the tumor necrosis factor receptor superfamily), and the receptor activator of nuclear factor-κB ligand (RANKL). When RANKL binds to RANKL receptor located on osteoclast precursor cells, it causes differentiation into mature osteoclasts and stimulates the multiple mechanisms by which bone resorption occurs. The osteoblast also secretes OPG that acts as a decoy receptor for RANKL, thus putting the brakes on osteoclasts. Also, T-cells play critical roles in maintaining bone mass. Estrogen deficiency of normal menopause causes T cells to secrete tumor necrosis factor-alpha and RANKL that activate osteoclasts causing bone resorption (18,19).
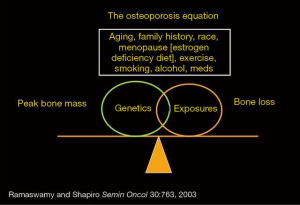
In healthy women, peak bone mass occurs around age 30 years. After 30 years age-related bone loss in both women and men occurs. The magnitude of bone loss in women (relative to men) is higher due to menopause, where estrogen levels may be decreased by one hundred-fold as compared with premenopausal women. A key point is everyone loses bone after age 30 years. Therefore, osteoporosis is an equation (Figure 3) (20) subtracting the loss of bone mass relating to aging and menopause from starting bone mass. Although modifiable risk factors include current smoking, excess alcohol consumption of greater than two drinks per day, and chronic steroid use, osteoporosis is primarily a genetic disease with the most influential non-modifiable risk factors being aging, parental history of non-traumatic fracture, low body mass (or weight under 150 pounds) and diabetes (21).
Genetics of AI-induced osteoporosis and AI-induced myalgias
The genetics of osteoporosis is complex involving single-nucleotide polymorphisms (SNPs) in twenty or more candidate genes (22,23). Several candidate gene SNPs specifically associate with AI-induced bone loss and even associate with response to AIs (24). In postmenopausal women with ER-positive breast cancers receiving AI, SNPs in the ESR1, ESR2, CYP19A1, and CYP11A1 predict decreases in bone density (25-27). The results of an extensive case-cohort genome-wide association study (GWAS) show that three SNPs in or near six genes located on chromosomes 20 (CTSZ, SLMO2, ATP5E), chromosome 6 (TRAM2, TRAM14A), and chromosome 2 (MAP4K4) relate to an increased risk of fractures in women receiving AIs (28). In preclinical experiments these six-genes are estrogen-regulated, and knocking down the expression of these genes increased the expression of genes known to associate with osteoporosis. Ideally, a blood test of germ-line DNA would identify SNPs that predict who has higher (or lower) risks of bone loss or fractures before starting AIs. If this were true, one could better tailor treatment either by changing therapy to tamoxifen, which mitigates bone loss in postmenopausal women or by initiating anti-resorptive drugs at same time as AI. However, the literature on this topic is conflicting based on studies that use different methodologies, sample sizes, populations, and other factors. In fact, a recent review concluded “…there are no strong associations between functional SNPs and AI-related adverse events with clinical implications.’’ (29).
Specific SNPs also associate with AI-induced arthralgia and myalgia. Statistically significant associations with SNPs in OPG, CYP17A1, vitamin D receptor (VDR), and CYP2 predict for higher risks of AI-induced adverse musculoskeletal events (30,31). However, more research is needed before SNPs are used in clinical decision-making (32).
Screening, risk factors, prevention, and treatment of AI-induced osteoporosis
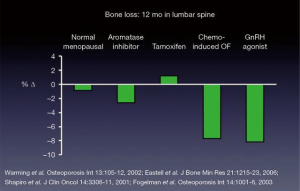
The detection, prevention, and treatment of osteoporosis fundamentally do not differ in women with and without breast cancer. Routine treatments for breast cancer like gonadotropin-releasing hormone agonists (33), chemotherapy-induced ovarian failure in premenopausal women (34), or AIs in postmenopausal women (6) lower estrogen levels and accelerate bone loss. Figure 4 (34-37) describes the relative magnitude of bone loss with different treatments. AIs cause two to three-fold higher bone loss than the rates observed in early postmenopausal bone loss.
Screening and risk factor assessment
The most common screening for osteoporosis is central dual-energy X-ray absorptiometry (DXA) scans measuring the total spine, hip, and femoral neck (10). The T-score is most crucial outcome variable of DXA scanning because it predicts fracture risk (38). The T-score is the number of standard deviations (SDs) by which bone mineral density (BMD) falls above or below the mean peak BMD for a reference population of healthy 20–29 years old females. For every 1 SD decrease in T-score, relative risk of fracture increases about 1.5 to 2.5-fold. The World Health Organization defines a normal T-score as −1.0 or above, T-scores between −1 and −2.5 as osteopenia, and T-scores below −2.5 or a non-traumatic fracture as osteoporosis (39). The Z score is another outcome variable of DXA scans that is the number of SDs the individual’s bone density falls above or below that of reference population of the same age and sex. The Z-score is useful for considering secondary causes of osteoporosis.
Women initiating AI treatment should receive a baseline DXA scan if they have never had one before or depending on the timing of the last DXA scan. Counseling should occur about modifiable risk factors that not only affect bone health but also influence overall health (e.g., current smoking, daily alcohol intake of three or more drinks, and increasing physical activity). Routinely checking vitamin 25-OH D levels is controversial. Vitamin D deficiency and insufficiency are prevalent among the general population and in women with breast cancer (40-42), especially in minority populations. Some health care providers routinely check a serum 25-OH vitamin D level before starting AIs. Others (including the authors), use the initial DXA scan as a guide, checking serum 25-OH vitamin D if osteopenia or osteoporosis is present.
Whether supplementing calcium and vitamin D will decrease non-traumatic fractures in postmenopausal women with or without osteoporosis is controversial (39). The most recent Cochrane review concludes that vitamin D alone does not have an impact on fractures (43). Supplementing calcium and vitamin D does lead to a small, statistically significant decrease in risk of hip but not vertebral fractures, and this is dependent on patient population (low versus high risk of osteoporosis) and the setting (outpatient versus institutionalized). However, there is evidence that supplemental calcium and vitamin D decreases postmenopausal BMD loss (39). More recently, there is evidence that calcium and vitamin D supplementation prevents falls by affecting muscle strength (44-46). Preventing fall is essential, as this will reduce fractures in an aging population of women with osteopenia or osteoporosis.
Relatively few trials have evaluated the impact of calcium and vitamin D supplementation in women receiving AI or cancer treatment-induced bone loss. Of those that did, the consensus is that doses of supplemental calcium and vitamin D of 500–1,500 mg and 200–1,000 IU, respectively, do not prevent loss of BMD (47) but mitigate it (45).
There is a broad consensus of policy-making organizations (e.g., National Osteoporosis Foundation, National Academy of Sciences, Institute of Medicine, and US Preventive Services Task Force) that women over age 50 years should receive 1,000–1,200 mg of calcium (including dietary calcium) and 800–1,000 IU vitamin D3 (cholecalciferol) daily (39). In addition to mitigating BMD loss, there is evidence to support other positive health outcomes, including cancer prevention (48). These doses of calcium and vitamin D supplementation are also the recommendations of several position papers on AI-induced bone loss (5,49).
Assessing fracture risk
The goal of evaluating fracture risk is preventing non-traumatic fractures of the hip and vertebrae. The primary endpoint is reducing fractures when testing osteoporosis drugs. In contrast, few studies have fractures as a primary endpoint in women with breast cancer receiving AIs. Most studies use a surrogate endpoint, BMD, as the primary endpoint and to make decisions when to institute anti-resorptive treatment. In breast cancer, trials are not large enough, and follow-up is too short to detect a reduction in fractures as a primary endpoint.
The fracture risk assessment (FRAX®) calculator is commonly used to estimate the ten-year risk of hip and non-hip vertebral fractures (www.sheffield.ac.uk/FRAX/) (21,50). FRAX® uses age, height, weight, sex, a variety risk factors (prior fracture, parental hip fracture, current smoking, glucocorticoids, rheumatoid arthritis, secondary osteoporosis, and alcohol of 3 or more drinks per day) and optional femoral neck T-score of BMD (51). The FRAX® calculator estimates the absolute ten-year risk of hip or non-hip fractures, but also the age-related thresholds for treating or not treating with anti-resorptive drugs. There are versions of FRAX® specific for each country.
FRAX® has limitations [described extensively in reference (21)]. The principal guideline groups around the world recently published a consensus statement on the management of AI-induced bone loss (49). In the guideline consensus paper, FRAX® may underestimate the ten-year fracture risk in women receiving AIs, and the authors provide an algorithm for using anti-resorptive drugs that don’t involve FRAX® (Figure 5) (49). This contrasts with National Comprehensive Cancer Center Network (NCCN) latest recommendations that suggest using FRAX® to estimate risks of hip and non-hip fractures (52).
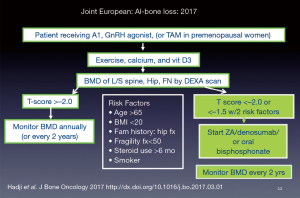
The trabecular bone score (TBS) is an assessment of bone microarchitecture derived from the DXA lumbar spine measurements. The TBS independently predicts falls and osteoporotic fractures in both men and women (53). In the study by Hans et al. incorporating TBS and BMD measurements was better than either alone in predicting osteoporotic fracture risk (54). A retrospective pilot trial of one hundred women with ER-positive breast cancers using BMD, FRAX®, and TBS measures at baseline (before starting AI) and two years post-AI treatment (55). The combination of three measurements identified a higher number of women at risk of osteoporotic fractures. Incorporating TBS into FRAX makes sense because it takes into consideration a measure of bone strength. There are not enough data yet to recommend incorporating TBS into routine fracture risk assessment in screening, either for osteoporosis or for women with an AI-induced bone loss.
Prevention and treatment with anti-resorptive drugs to prevent fractures in women receiving AIs
Table 1 describes the fracture rates in the major clinical trials of AI versus tamoxifen. All AI-treatment groups had statistically significant increases fractures relative to tamoxifen (12,56-60). The fractures remain elevated during the five years of AI treatment and decreases to the same rate as tamoxifen-treated patients during years five to ten (12).

Full table
The osteoclast inhibitor, zoledronic acid (ZA), and the RANKL inhibitor, denosumab, are the two drugs used to mitigate BMD loss in women receiving women receiving AIs (5,6,49). Table 2 describes ZA and denosumab. The primary differences in these drugs are in their mechanism of actions, routes of administration, toxicities, and the costs (61). There are no randomized trials directly comparing ZA and denosumab to mitigate BMD loss in women treated with AIs. More recently, a randomized placebo-controlled trial of denosumab 60 mg subcutaneously every six months in postmenopausal women receiving AI was first to show a statistically significant reduction in fractures (62).
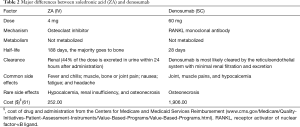
Full table
Instructive are the results of the ZO-Fast trial (63). Over 1,000 postmenopausal women receiving letrozole 2.5 mg a day were randomized to receive either immediate ZA 4 mg IV every six months for five years or to receive ZA only if the DXA showed a decreasing T-score of less than or equal −2.0, or an osteoporotic fracture occurred. Entry criteria included those women with normal BMD (i.e., T-scores of −1.0 or above) or osteopenia with T-scores of −1.0 to −2.0. As expected the group that was randomized to immediate ZA statistically significantly increased BMD in lumbar while the delayed ZA lost bone. However, after five years, only 27% of delayed ZA group received ZA. Thus, only minority of women on AIs needed anti-resorptive drugs, at least for first five years.
Anti-resorptive drugs as anti-cancer drugs
Several randomized trials (64-66) and a recent meta-analysis (67) suggest that in postmenopausal women or premenopausal women rendered postmenopausal with a GnRH agonist (64), ZA improves clinical outcomes such as distant recurrence, bone recurrence, and cancer deaths. Included in the meta-analysis were over 11,000 postmenopausal and over 6,000 premenopausal early stage women with breast cancer. In the postmenopausal women only, the ten-year absolute decreases in distant recurrence, bone recurrence, and cancer mortality were 1.5% (P=0.10), 2.2% (P=0.0002), and 3.3% (P=0.002), respectively. In 2017, the Canadian Care Ontario and American Society of Clinical Oncology Clinical Practice Guidelines Focused Update put out a statement on the use adjuvant anti-resorptive drugs. The guideline stated “consider” ZA (4 mg intravenously) every six months for three to five years or oral clodronate 1,600 mg per day (only available in Europe) for three years in high-risk postmenopausal women (68). There is insufficient evidence at present to recommend denosumab as anti-cancer drug. Additional clinical trials of anti-resorptive drugs specifically in early stage postmenopausal women are needed to confirm the preliminary evidence of the meta-analysis (69).
AI-induced arthralgia and myalgia
AIs cause musculoskeletal systems including joint pain (arthralgias) and muscle pain (myalgias). Aromatase-induced musculoskeletal symptoms include both arthralgias and myalgias. For the purposes of this review, the reference to AI-induced arthralgias, will often include myalgias, depending on the particular study. AI-induced arthralgia is one of the most common side effects and is a significant contributor to nonadherence and discontinuation of drug (discussed below). The primary criteria that define AI-induced arthralgia are joint pain that develops or worsens while taking an AI, stopping the AI for two weeks and joint pain resolves, and then restarting the AI and joint pain returns (70). Minor criteria include pain affecting the joints symmetrically, pain in the hands or wrists, carpal tunnel syndrome, decreased grip strength, morning stiffness, and improvement with exercise. AI-induced arthralgia tends to occur soon after initiating AI with median onset 1.6 months (range 0.4 to 10 months) (71).
The prevalence of AI-induced arthralgias is about 50% (range 20% to 74%) in a recent systemic review and a meta-analysis (9). In retrospective analyses, independent predictors that increase the risk of developing AI-induce arthralgias include prior taxane use (as opposed to non-taxane containing chemotherapy), and previous anti-estrogen treatment (as opposed to no prior anti-estrogen therapy), whereas independent predictors of decreased risk are body mass index (BMI) 25–30 (as opposed BMI under 25 or over 30), and stage III breast cancer (as opposed to stage I cancers) (9). In a prospective trial of three hundred ninety-two postmenopausal women receiving anastrozole, independent risk factors for AI-induced arthralgia were shorter time since menopause and prior adjuvant chemotherapy (72). BMI was not a risk factor in this study. The wide variation in the prevalence of AI-induced arthralgias, and the uncertainty of impact of risk factors (e.g., BMI), is in part related to retrospective study designs in majority of the studies, varying definitions of AI-induced arthralgia, as well as the instruments used to assess this side-effect.
The mechanism of AI-induced arthralgia is poorly understood, and there are several hypotheses (70). The MRI may show tenosynovitis with enhancement and thickening of tendon sheaths consistent with an inflammatory process (73,74). One theory invokes suppression of pro-inflammatory cytokines [i.e., interleukin-6 (IL-6)] by the activity of synovial cell aromatase that produces estrogen from androgens. With AI treatment, decreased estrogen levels result in increased levels of IL-6, which causes inflammation and tenosynovitis (70).
AI-induced arthralgia is a significant contributor to nonadherence and drug discontinuation (8). The definition and causes of nonadherence are reviewed in Hugtenburg et al. (75). The rates of nonadherence and discontinuation increase over time. In one retrospective study of over 2,000 women receiving care in single health system treated with anti-estrogens (i.e., either tamoxifen, anastrozole, or switching between them), 21% were non-adherent by the end of one year, and after five years 73% were non-adherent (76). There are reports by others of similar non-adherence rates (77-80).
Non-adherence may affect worsening disease-free survival and increases cancer mortality (81,82). Independent risk factors for nonadherence include symptoms (83), and higher co-pays and out of pocket costs (84,85) and negative health beliefs (83,86), Also, poor doctor-patient communication, lack of social support, perceptions of low risk or recurrence, race (i.e., African-American), and older and younger ages (87).
Treatments for AI-induced arthralgia
There are several systemic reviews and meta-analyses on treatments AI-induced arthralgia (88,89). The interventions to treat AI-induced arthralgia include drugs, nutritional supplements, acupuncture, relaxation techniques, and physical therapies (including exercise, yoga, tai chi, and walking). Several caveats are important to point out when reviewing interventions for AI-induced arthralgia. The primary outcome is pain in the most studies. The sample size of the majority of studies is one-hundred women or less, and only six of thirty-eight (16%) enrolled randomized controlled trials of more than one hundred women (88). Studies vary in design including randomized controlled trials, prospective cohorts, and retrospective. Also, the entry criteria, and especially the validated instruments, vary greatly between studies. The preceding factors affect study quality, and thus the evidence for the interventions is weak to moderate (90).
One of the simplest and most effective interventions is switching an AI for another or switching to tamoxifen (91,92). About one-third of women will switch to another AI and be able to tolerate the second AI for a median of nearly 14 months (3 to 39 months) (93). One randomized trial of vitamin D3 at 4,000 IU versus 600 IU per day (94), and a second in which women on 600 IU per day plus 1,200 mg/day calcium for a run-in period were then randomized to 30,000 IU vitamin D3 per week versus placebo (95) have been conducted. In the two trials, measures of joint pain, stiffness, or functional ability were not statistically significant between the groups that received the higher vitamin D dosing. A recent randomized placebo-controlled trial of duloxetine in two hundred ninety-nine early-stage postmenopausal women receiving AI with at least moderate joint pains showed statistically significant improvements joint pain, stiffness, pain interference, and physical functions (96). Another randomized, placebo-controlled trial of omega-3 fatty acids in two hundred forty-nine women with similar characteristics to the duloxetine trial showed no statistically significant differences in pain (97). Interestingly, women randomized to placebo experienced more than fifty percent pain relief.
The results of small-randomized trials of acupuncture are conflicting (89). The results in randomized exercise intervention (twice-weekly resistance and supervised aerobic training for twelve months) versus usual care in one hundred twenty-one early stage women with breast cancer receiving AI with least mild joint pain show statistically significant reductions in pain, pain severity, and pain interference, and other validated instruments (98).
There is pressing need for interventions that mitigate AI-induced arthralgia that are cost-effective and feasible outside the resource-rich Cancer Center and in the community where the majority of women receive treatment for breast cancer. The emphasis should be on randomized controlled trials adequately powered to detect a statistically and clinically significant effect sizes, using standardized instruments to facilitate cross-trial comparisons.
Suggested approach to AI-induced arthralgias
Before starting AI, weight management and increasing physical activity are part of the routine care for every woman with breast cancer. Increasing physical activity has a wealth of benefits beyond breast cancer, and may mitigate AI-induced arthralgia. It is beyond the scope of this review to discuss all of the “teachable moments” (99), and the ways to affect behavior modification. However, women receiving AIs desire information from their oncology provider about AI-induced arthralgia, in addition to other side effects, and are more likely to increase physical activity if their oncologist recommends it (100).
Before discussing the side effects of AIs, two points deserve emphasis: (I) there are women for whom no to minimal symptoms occur (101), and (II) if symptoms occur there are approaches to mitigate them. If AI-arthralgia is suspected, one can stop the AI for 2–3 weeks. If the joint pain improves, re-challenge with same AI can be considered. If the joint pain reoccurs, options include changing the AI. If the joint pain does not resolve with stopping, it is not likely related to the specific use of AI. A referral to acupuncture, despite conflicting data, will work in some women and allow them to stay on AI. Although it may be the result of a placebo effect, from the individual's point of view, this of no importance (102). Another option is starting duloxetine.
Some women are intolerant of all three AIs. In this case, switching to tamoxifen is option of choice if there are no contraindications to this drug.
Conclusions
AIs are mainstays in the treatment of postmenopausal women with ER-positive and will be for the foreseeable future. AI-induced arthralgias are common side effects and responsible for nonadherence and discontinuation. Interventions for AI-induced arthralgias are many, but many trials are underpowered and use different instruments in assessing the primary endpoint of joint pain. Switching the AI is helpful in about 30% of women and is simple to do. More research is needed to understand the mechanisms of AI-induced arthralgia, SNPs that predict that risk, and interventions that are feasible and cost-effective.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Binbing Y, Tiwari RC, Feuer EJ. Estimating the personal cure rate of cancer patients using population-based grouped cancer survival data. Stat Methods Med Res 2011;20:261-74. [Crossref] [PubMed]
- Shapiro CL, Jacobsen PB, Henderson T, et al. ReCAP: ASCO Core Curriculum for Cancer Survivorship Education. J Oncol Pract 2016;12:145. [Crossref] [PubMed]
- Milat F, Vincent AJ. Management of bone disease in women after breast cancer. Climacteric 2015;18 Suppl 2:47-55. [Crossref] [PubMed]
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [Crossref] [PubMed]
- Tremollieres FA, Ceausu I, Depypere H, et al. Osteoporosis management in patients with breast cancer: EMAS position statement. Maturitas 2017;95:65-71. [Crossref] [PubMed]
- Cepa M, Vaz C. Management of bone loss in postmenopausal breast cancer patients treated with aromatase inhibitors. Acta Reumatol Port 2015;40:323-30. [PubMed]
- Hershman DL, Loprinzi C, Schneider BP. Symptoms: Aromatase Inhibitor Induced Arthralgias. Adv Exp Med Biol 2015;862:89-100. [Crossref] [PubMed]
- Lombard JM, Zdenkowski N, Wells K, et al. Aromatase inhibitor induced musculoskeletal syndrome: a significant problem with limited treatment options. Support Care Cancer 2016;24:2139-46. [Crossref] [PubMed]
- Beckwee D, Leysen L, Meuwis K, et al. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: a systematic review and meta-analysis. Support Care Cancer 2017;25:1673-86. [Crossref] [PubMed]
- Pant S, Shapiro CL. Aromatase inhibitor-associated bone loss: clinical considerations. Drugs 2008;68:2591-600. [Crossref] [PubMed]
- Lonning PE, Eikesdal HP. Aromatase inhibition 2013: clinical state of the art and questions that remain to be solved. Endocr Relat Cancer 2013;20:R183-201. [Crossref] [PubMed]
- Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 2010;11:1135-41. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341-52. [Crossref] [PubMed]
- Riemsma R, Forbes CA, Kessels A, et al. Systematic review of aromatase inhibitors in the first-line treatment for hormone sensitive advanced or metastatic breast cancer. Breast Cancer Res Treat 2010;123:9-24. [Crossref] [PubMed]
- Gennari L, Merlotti D, Nuti R. Aromatase activity and bone loss. Adv Clin Chem 2011;54:129-64. [Crossref] [PubMed]
- Folkestad L, Bjarnason NH, Bjerregaard JK, et al. The effect of aromatase inhibitors on bone metabolism. Basic Clin Pharmacol Toxicol 2009;104:3-10. [Crossref] [PubMed]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003;423:337-42. [Crossref] [PubMed]
- Weitzmann MN, Pacifici R. T cells: unexpected players in the bone loss induced by estrogen deficiency and in basal bone homeostasis. Ann N Y Acad Sci 2007;1116:360-75. [Crossref] [PubMed]
- D'Amelio P, Grimaldi A, Di Bella S, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone 2008;43:92-100. [Crossref] [PubMed]
- Ramaswamy B, Shapiro CL. Osteopenia and osteoporosis in women with breast cancer. Semin Oncol 2003;30:763-75. [Crossref] [PubMed]
- Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 2013;24:23-57. [Crossref] [PubMed]
- Urano T, Inoue S. Recent genetic discoveries in osteoporosis, sarcopenia and obesity. Endocr J 2015;62:475-84. [Crossref] [PubMed]
- Perez-Castrillon JL, Olmos JM, Nan DN, et al. Polymorphisms of the WNT10B gene, bone mineral density, and fractures in postmenopausal women. Calcif Tissue Int 2009;85:113-8. [Crossref] [PubMed]
- Artigalas O, Vanni T, Hutz MH, et al. Influence of CYP19A1 polymorphisms on the treatment of breast cancer with aromatase inhibitors: a systematic review and meta-analysis. BMC Med 2015;13:139. [Crossref] [PubMed]
- Napoli N, Rastelli A, Ma C, et al. Genetic polymorphism at Val80 (rs700518) of the CYP19A1 gene is associated with aromatase inhibitor associated bone loss in women with ER + breast cancer. Bone 2013;55:309-14. [Crossref] [PubMed]
- Oesterreich S, Henry NL, Kidwell KM, et al. Associations between genetic variants and the effect of letrozole and exemestane on bone mass and bone turnover. Breast Cancer Res Treat 2015;154:263-73. [Crossref] [PubMed]
- Rodriguez-Sanz M, Garcia-Giralt N, Prieto-Alhambra D, et al. CYP11A1 expression in bone is associated with aromatase inhibitor-related bone loss. J Mol Endocrinol 2015;55:69-79. [PubMed]
- Liu M, Goss PE, Ingle JN, et al. Aromatase inhibitor-associated bone fractures: a case-cohort GWAS and functional genomics. Mol Endocrinol 2014;28:1740-51. [Crossref] [PubMed]
- Sini V, Botticelli A, Lunardi G, et al. Pharmacogenetics and aromatase inhibitor induced side effects in breast cancer patients. Pharmacogenomics 2017;18:821-30. [Crossref] [PubMed]
- Lintermans A, Van Asten K, Jongen L, et al. Genetic variant in the osteoprotegerin gene is associated with aromatase inhibitor-related musculoskeletal toxicity in breast cancer patients. Eur J Cancer 2016;56:31-6. [Crossref] [PubMed]
- Garcia-Giralt N, Rodriguez-Sanz M, Prieto-Alhambra D, et al. Genetic determinants of aromatase inhibitor-related arthralgia: the B-ABLE cohort study. Breast Cancer Res Treat 2013;140:385-95. [Crossref] [PubMed]
- Borrie AE, Kim RB. Molecular basis of aromatase inhibitor associated arthralgia: known and potential candidate genes and associated biomarkers. Expert Opin Drug Metab Toxicol 2017;13:149-56. [Crossref] [PubMed]
- Tremollieres FA. Screening for osteoporosis after breast cancer: for whom, why and when. Maturitas 2014;79:343-8. [Crossref] [PubMed]
- Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol 2001;19:3306-11. [Crossref] [PubMed]
- Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int 2002;13:105-12. [Crossref] [PubMed]
- Eastell R, Hannon RA, Cuzick J, et al. Effect of an aromatase inhibitor on bmd and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res 2006;21:1215-23. [Crossref] [PubMed]
- Fogelman I, Blake GM, Blamey R, et al. Bone mineral density in premenopausal women treated for node-positive early breast cancer with 2 years of goserelin or 6 months of cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Osteoporos Int 2003;14:1001-6. [Crossref] [PubMed]
- Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA 2002;288:1889-97. [Crossref] [PubMed]
- Kling JM, Clarke BL, Sandhu NP. Osteoporosis prevention, screening, and treatment: a review. J Womens Health (Larchmt) 2014;23:563-72. [Crossref] [PubMed]
- Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res 2011;31:48-54. [Crossref] [PubMed]
- Friedman CF, DeMichele A, Su HI, et al. Vitamin d deficiency in postmenopausal breast cancer survivors. J Womens Health (Larchmt) 2012;21:456-62. [Crossref] [PubMed]
- Nogues X, Servitja S, Pena MJ, et al. Vitamin D deficiency and bone mineral density in postmenopausal women receiving aromatase inhibitors for early breast cancer. Maturitas 2010;66:291-7. [Crossref] [PubMed]
- Avenell A, Mak JC, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev 2014. [PubMed]
- Wu H, Pang Q. The effect of vitamin D and calcium supplementation on falls in older adults: A systematic review and meta-analysis. Orthopade 2017;46:729-36. [Crossref] [PubMed]
- Dawson-Hughes B. Vitamin D and muscle function. J Steroid Biochem Mol Biol 2017;173:313-6. [Crossref] [PubMed]
- Dhaliwal R, Aloia JF. Effect of Vitamin D on Falls and Physical Performance. Endocrinol Metab Clin North Am 2017;46:919-33. [Crossref] [PubMed]
- Datta M, Schwartz GG. Calcium and vitamin D supplementation and loss of bone mineral density in women undergoing breast cancer therapy. Crit Rev Oncol Hematol 2013;88:613-24. [Crossref] [PubMed]
- Jeon SM, Shin EA. Exploring vitamin D metabolism and function in cancer. Exp Mol Med 2018;50:20. [Crossref] [PubMed]
- Hadji P, Aapro MS, Body JJ, et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol 2017;7:1-12. [Crossref] [PubMed]
- Kanis JA, McCloskey EV, Johansson H, et al. Development and use of FRAX in osteoporosis. Osteoporos Int 2010;21 Suppl 2:S407-13. [Crossref] [PubMed]
- Leslie WD, Majumdar SR, Lix LM, et al. High fracture probability with FRAX usually indicates densitometric osteoporosis: implications for clinical practice. Osteoporos Int 2012;23:391-7. [Crossref] [PubMed]
- Gralow JR, Biermann JS, Farooki A, et al. NCCN Task Force Report: Bone Health in Cancer Care. J Natl Compr Canc Netw 2013;11 Suppl 3:S1-50. [Crossref] [PubMed]
- McCloskey EV, Oden A, Harvey NC, et al. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J Bone Miner Res 2016;31:940-8. [Crossref] [PubMed]
- Hans D, Goertzen AL, Krieg MA, et al. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 2011;26:2762-9. [Crossref] [PubMed]
- Mariotti V, Page DB, Davydov O, et al. Assessing fracture risk in early stage breast cancer patients treated with aromatase-inhibitors: An enhanced screening approach incorporating trabecular bone score. J Bone Oncol 2016;7:32-7. [Crossref] [PubMed]
- Rabaglio M, Sun Z, Price KN, et al. Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1-98 trial. Ann Oncol 2009;20:1489-98. [Crossref] [PubMed]
- van de Velde CJ, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet 2011;377:321-31. [Crossref] [PubMed]
- Goss PE, Ingle JN, Pater JL, et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol 2008;26:1948-55. [Crossref] [PubMed]
- Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 2005;366:455-62. [Crossref] [PubMed]
- Dubsky PC, Jakesz R, Mlineritsch B, et al. Tamoxifen and anastrozole as a sequencing strategy: a randomized controlled trial in postmenopausal patients with endocrine-responsive early breast cancer from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol 2012;30:722-8. [Crossref] [PubMed]
- Shapiro CL, Moriarty JP, Dusetzina S, et al. Cost-Effectiveness Analysis of Monthly Zoledronic Acid, Zoledronic Acid Every 3 Months, and Monthly Denosumab in Women With Breast Cancer and Skeletal Metastases: CALGB 70604 (Alliance). J Clin Oncol 2017;35:3949-55. [Crossref] [PubMed]
- Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015;386:433-43. [Crossref] [PubMed]
- Coleman R, de Boer R, Eidtmann H, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol 2013;24:398-405. [Crossref] [PubMed]
- Gnant M, Mlineritsch B, Stoeger H, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol 2015;26:313-20. [Crossref] [PubMed]
- Coleman RE, Marshall H, Cameron D, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med 2011;365:1396-405. [Crossref] [PubMed]
- Paterson AH, Anderson SJ, Lembersky BC, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol 2012;13:734-42. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative G, Coleman R, Powles T, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 2015;386:1353-61. [Crossref] [PubMed]
- Dhesy-Thind S, Fletcher GG, Blanchette PS, et al. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2017;35:2062-81. [Crossref] [PubMed]
- O'Carrigan B, Wong MH, Willson ML, et al. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev 2017;10. [PubMed]
- Niravath P. Aromatase inhibitor-induced arthralgia: a review. Ann Oncol 2013;24:1443-9. [Crossref] [PubMed]
- Henry NL, Giles JT, Ang D, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 2008;111:365-72. [Crossref] [PubMed]
- Egawa C, Hirokaga K, Takao S, et al. Risk factors for joint symptoms in postmenopausal Japanese breast cancer patients treated with anastrozole: a prospective multicenter cohort study of patient-reported outcomes. Int J Clin Oncol 2016;21:262-9. [Crossref] [PubMed]
- Morales L, Pans S, Verschueren K, et al. Prospective study to assess short-term intra-articular and tenosynovial changes in the aromatase inhibitor-associated arthralgia syndrome. J Clin Oncol 2008;26:3147-52. [Crossref] [PubMed]
- Lintermans A, Laenen A, Van Calster B, et al. Prospective study to assess fluid accumulation and tenosynovial changes in the aromatase inhibitor-induced musculoskeletal syndrome: 2-year follow-up data. Ann Oncol 2013;24:350-5. [Crossref] [PubMed]
- Hugtenburg JG, Timmers L, Elders PJ, et al. Definitions, variants, and causes of nonadherence with medication: a challenge for tailored interventions. Patient Prefer Adherence 2013;7:675-82. [Crossref] [PubMed]
- Nekhlyudov L, Li L, Ross-Degnan D, et al. Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat 2011;130:681-9. [Crossref] [PubMed]
- Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 2008;26:556-62. [Crossref] [PubMed]
- Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol 2009;20:431-6. [Crossref] [PubMed]
- Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat 2011;125:191-200. [Crossref] [PubMed]
- Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 2010;28:4120-8. [Crossref] [PubMed]
- Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 2011;126:529-37. [Crossref] [PubMed]
- Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment Adherence and Its Impact on Disease-Free Survival in the Breast International Group 1-98 Trial of Tamoxifen and Letrozole, Alone and in Sequence. J Clin Oncol 2016;34:2452-9. [Crossref] [PubMed]
- Simon R, Latreille J, Matte C, et al. Adherence to adjuvant endocrine therapy in estrogen receptor-positive breast cancer patients with regular follow-up. Can J Surg 2014;57:26-32. [Crossref] [PubMed]
- Tan X, Marshall VD, Anderson RT, et al. Adjuvant therapy use among Appalachian breast cancer survivors. Medicine (Baltimore) 2015;94. [Crossref] [PubMed]
- Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol 2011;29:2534-42. [Crossref] [PubMed]
- Brier MJ, Chambless DL, Gross R, et al. Perceived barriers to treatment predict adherence to aromatase inhibitors among breast cancer survivors. Cancer 2017;123:169-76. [Crossref] [PubMed]
- Chlebowski RT, Kim J, Haque R. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila) 2014;7:378-87. [Crossref] [PubMed]
- Roberts K, Rickett K, Greer R, et al. Management of aromatase inhibitor induced musculoskeletal symptoms in postmenopausal early Breast cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2017;111:66-80. [Crossref] [PubMed]
- Yang GS, Kim HJ, Griffith KA, et al. Interventions for the Treatment of Aromatase Inhibitor-Associated Arthralgia in Breast Cancer Survivors: A Systematic Review and Meta-analysis. Cancer Nurs 2017;40:E26-41. [Crossref] [PubMed]
- Flecha OD, Douglas de Oliveira DW, Marques LS, et al. A commentary on randomized clinical trials: How to produce them with a good level of evidence. Perspect Clin Res 2016;7:75-80. [Crossref] [PubMed]
- Briot K, Tubiana-Hulin M, Bastit L, et al. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Cancer Res Treat 2010;120:127-34. [Crossref] [PubMed]
- Kwan ML, Roh JM, Laurent CA, et al. Patterns and reasons for switching classes of hormonal therapy among women with early-stage breast cancer. Cancer Causes Control 2017;28:557-62. [Crossref] [PubMed]
- Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 2012;30:936-42. [Crossref] [PubMed]
- Shapiro AC, Adlis SA, Robien K, et al. Randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Res Treat 2016;155:501-12. [Crossref] [PubMed]
- Khan QJ, Kimler BF, Reddy PS, et al. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms in women with breast cancer receiving adjuvant letrozole. The VITAL trial. Breast Cancer Res Treat 2017;166:491-500. [Crossref] [PubMed]
- Henry NL, Unger JM, Schott AF, et al. Randomized, Multicenter, Placebo-Controlled Clinical Trial of Duloxetine Versus Placebo for Aromatase Inhibitor-Associated Arthralgias in Early-Stage Breast Cancer: SWOG S1202. J Clin Oncol 2018;36:326-32. [Crossref] [PubMed]
- Hershman DL, Unger JM, Crew KD, et al. Randomized Multicenter Placebo-Controlled Trial of Omega-3 Fatty Acids for the Control of Aromatase Inhibitor-Induced Musculoskeletal Pain: SWOG S0927. J Clin Oncol 2015;33:1910-7. [Crossref] [PubMed]
- Irwin ML, Cartmel B, Gross CP, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol 2015;33:1104-11. [Crossref] [PubMed]
- Bluethmann SM, Basen-Engquist K, Vernon SW, et al. Grasping the 'teachable moment': time since diagnosis, symptom burden and health behaviors in breast, colorectal and prostate cancer survivors. Psychooncology 2015;24:1250-7. [Crossref] [PubMed]
- Nyrop KA, Callahan LF, Rini C, et al. Aromatase inhibitor associated arthralgia: the importance of oncology provider-patient communication about side effects and potential management through physical activity. Support Care Cancer 2016;24:2643-50. [Crossref] [PubMed]
- Nestoriuc Y, von Blanckenburg P, Schuricht F, et al. Is it best to expect the worst? Influence of patients' side-effect expectations on endocrine treatment outcome in a 2-year prospective clinical cohort study. Ann Oncol 2016;27:1909-15. [Crossref] [PubMed]
- Chavarria V, Vian J, Pereira C, et al. The Placebo and Nocebo Phenomena: Their Clinical Management and Impact on Treatment Outcomes. Clin Ther 2017;39:477-86. [Crossref] [PubMed]