Metabolic syndrome in breast cancer
Introduction
Breast cancer (BC) ranks first in cancer incidence and mortality worldwide, is a heterogeneous disease, progressive, which has been classified by histopathological features in two groups, in situ and invasive or infiltrating ductal carcinoma is the most frequent 70-80% and specific subtypes (tubular, colloid, medullary, lobular and papillary) more favorable prognosis. The anatomical extent of the disease is classified according to the TNM system that divided in different clinical stages that correlate with Survival rate, after which he joined the degree of nuclear differentiation and histopathological prognostic purposes, as well as other factors (such as lymphovascular invasion and proliferative markers) moderate utility (1,2). Incorporating new molecular prognostic markers as hormone receptors, estrogen receptor (ER) and the over-expression of the receptor gene from human epidermal growth factor 2 (HER-2neu) along with the development of targeted therapies and improve the predictive capability forecast incorporating new molecular factors. These two markers are essential to characterize the BC according to the presence or absence of hormonal receptors for estrogen and progesterone and HER-2neu, one type of receptor tyrosine kinase protein that is involved in normal growth cells and is found in some types of cancer cells, their presence help therapeutic management. the BC 75% to 80% are hormone receptor-positive, 15% to 20% HER-2neu positive and 10% to 20%, are Cancer Breast Triple Negative (BCTN) is defined by hormone receptor and HER2-neu negative and diagnosis is by exclusion, 85% of BCTN are basal or basaloid type, the basaloid type are triple negative, but not all triple negative are basaloid type, of infiltrating BCTN is reported, in order of frequency: ductal (68.4-86%), medullary (5.3-5.8%), lobular (2.9-1%), cribriform (0-0.9%), mucinous (0-2%) and papillary (0.6-0.9%), more than half of the BCTN larger than 2 cm, which is related with a late diagnosis are aggressive and undifferentiated or Grade 3 (G3), with positive axillary lymph nodes (N+), high rates of mitotic division and half of these tumors have mutations in the gene tumor suppressor protein 53 (p53), has a higher percentage of recurrences, distant metastases to visceral organs such as liver, lung and central nervous system primarily, and increased mortality (3); when compared with other subtypes of BM, are common in premenopausal women, especially African-American descent (1,3-6), with mutations in the over-expression of BRCA-1, which is characterized by:
(I) Upregulation of cytokeratins 5, 14, and 17, which are intermediate filaments that form part of the cytoskeleton of epithelial cells and comprise a complex multigenic family of related proteins, which are expressed in simple epithelia (cytokeratin 7, 8, 18, 19 and 20) and stratified epithelia (cytokeratins 1, 2, 5, 9, 10, 11, 16) are linked with specific cell differentiation, normal basal cells express cytokeratin high molecular weight, such as 5/6, 14, 17 and p63 protein, many of which also express CD117 (c-kit), which are responsible for the protection of the epithelium to mechanical stress act as signaling platforms forming intra-and intercellular connections and with the help of desmosomes, involved in regulation of cell proliferation and size, because there are changes in its expression in tissues in differentiation, injured and metastasis.
(II) Elevation receptor epidermal growth factor (EGFR) membrane plays a role in carcinogenesis, when activated controls cell proliferation, apoptosis, angiogenesis and metastasis development in most epithelial cancers (2). The BCTN dominates the basal-B (BCBL) and a difference between the two subtypes is a genetic level. Other molecular subtypes of BM are defined by their patterns of gene expression and include the BM luminal A, luminal B and BM group most over-expression of HER-2neu and claudins, a family of proteins that play a central role in the homeostasis mediated tight junctions that hold the barrier function and polarity of the cell, the correct expression and localization in various epithelia claudins play a role during tumor progression, are useful in diagnosing and treating cancer, decreased expression claudin 1 leads to an increase in apoptosis and claudins 1, 2, 3, 4, and 5 are incorporated in and promote the activation metalloproteinase acting on the development of invasion and metastasis in BCTN (1,3-6).
Since its first description of the molecular characteristics of BCTN in 2005, has become an active area of research (7-9). While initial studies focused on the molecular and clinical characterization of patients with this diagnosis, recent studies have identified subgroups of patients with BCTN, with proposals on molecular mechanisms that contribute to carcinogenesis and therapeutic interventions for patients. In this paper we review the studies on the link between the BM mainly BCTN and its association with metabolic syndrome (MS), type 2 diabetes mellitus (T2DM) and its management, which consists of central obesity, insulin resistance, impaired glucose, dyslipidemia and systemic hypertension (8).
Risk factors association with breast cancer
Obesity is associated with insulin resistance with T2DM, which are risk factors (RF) for cancer, a meta-analysis of body mass index (BMI) is associated with increased incidence of BM in postmenopausal women, and colon cancer, endometrial cancer, esophageal cancer, gallbladder cancer, pancreatic cancer, renal cancer, thyroid cancer, leukemia, multiple myeloma, prostate cancer and non-Hodgkin lymphoma but decreased (8,9) lung cancer. In premenopausal women with BM is less clear this devastation by differences in RF, Asian women if this association (OR, 95% CI, 1.01-1.32), while the relationship is reversed in North American women (OR, 95% CI, 0.85 to 0.98) and European women (OR, 95% CI, 0.84 to 0.94), suggesting that BMI is not an ideal measure to assess adiposity and other measures such as the waist-hip ratio (WHR) or waist circumference are specific measures to assess central or abdominal adiposity and preferred to assess the risk of cancer, another meta-analysis that examined the correlation between increased weight and risk of BM in premenopausal women tube positive association (10) and another report was associated with 79% (OR, 95% CI, 1.22-2.62) increased risk of BM in premenopausal women and 50% (95% CI, 1.10 to 2.4) in postmenopausal women. Similarly, another study reported a small association but with 37% lower risk (95% CI, 0.45 to 0.88) in premenopausal women (10) after adjusting for BMI. It can be concluded that overall obesity does not change the risk, but if central obesity increases the risk in premenopausal and general obesity and central obesity does not increase cancer risk in postmenopausal women, suggesting that insulin resistance and growth factors, insulin-like associated with central obesity, play a greater role in the risk of BM in premenopausal estrogen and a more important role in BM in postmenopausal women (10).
The association between obesity and incidence of all types of BC is established, when the index is compared in Waist Hip Size Women high (≥0.84) with short stature (
Women with T2DM have 20% greater risk of developing BM (11) (95% CI, 1.12-1.28), and women with BCTN, 58% had a diagnosis of MS compared with 37% of women with other types of BM if using the criteria of national education program on cholesterol, and when compared with the criteria of the American Association of Clinical Endocrinologists (12), 52% of women with BCTN have MS 34% compared with other types BM, also reported a 75% higher risk of BC in postmenopausal women (95% CI, 1.37 to 2.22) that had at least three of the four components of MS, however, there higher prevalence of T2DM in BCTN when compared with other subtypes of BM.
Recently, dyslipidemia and hypertension associated with risk of BM and reported a positive association between total cholesterol and risk of BM (13) (OR, 95% CI, 1.03 to 1.33) patients was compared with total cholesterol ≥240 mg/dL and 14,15), however, after adjusting for other factors such as BMI, the risk was not significant (95% CI, 0.93-1.40). Epidemiological studies suggest a positive association between MS as a whole, along with many of its individual components and risk of BM. Multiple confounders that may mediate this effect must be considered in order to determine if this is a causal effect on subpopulations of patients with BCTN (e.g., postmenopausal women vs. premenopausal) having unique molecular and clinical characteristics.
Recurrence risk and mortality in breast cancer
In addition to the RF that influence breast cancer incidence (primary prevention), it is essential to understand which factors influence BM recurrence (secondary prevention). When compared with other subtypes of BCTN BM, BCTN have adverse prognostic indicators that affect morbidity and mortality rate also has a higher rate of recurrence compared with other subtypes of BM, especially within the first five years after diagnosis (15) even after five years, the risk of recurrence is greatly reduced.
Obese patients with BM have higher recurrence and worse prognosis compared with thin patients, the increase in BMI was significantly associated with increased mortality rates (16) BM, compared with the group of women with lower BMI (18.5-24.9), mortality rate increases BM, 34% for BMI 25.0 to 29.9 (95% CI, 1.23 to 1.46), 63% for a BMI of 30.0 to 34.9 (95% CI, 1.44-1.85), 70% for a BMI of 35.0 to 39.9 (RR=1.70, 95% CI, 1.33 to 2.17) and 112% for a BMI ≥40.0 (95%, 1.41 to 3.19), even women with early BM, BMI at diagnosis correlates with the prognosis of the disease and patients with BMI ≥30 kg/m2 had 46% higher risk of having distant metastases (95% CI, 1.11, 1.92) after 10 years and 38% more likely to die from BM (95% CI, 1.11-1.71) compared to patients with BMI 2 Chemotherapy (Qt) and adjuvant hormone therapy are less effective in patients with BMI >30, it is unknown whether this effect is mediated by poor response to Qt or differences in tumor biology (17). Although obese patients are more likely to have advanced BM, obesity continues to be an independent prognostic factor after controlling for other factors. Measuring (18) BMI, number of recurrences and mortality after diagnosis of BCTN and control other clinically relevant factors, there is no relationship between BMI and Survival rate (95% CI, 0.54 to 1.64) or disease-free (95% CI, 0.49 to 1.34). In patients diagnosed with BM, the overall relationship between obesity and response to neoadjuvant chemotherapy groups were compared (19) overweight (BMI between 25 and 2) and obesity (BMI ≥30 kg/m2) in weight groups normal/underweight (BMI <25) complete pathologic response to neoadjuvant chemotherapy was significant (95% CI, 0.45-0.99) in the normal/underweight compared to women with higher BMI, in addition to the overall survival rate is but, the studies comparing the risk of recurrence and mortality in patients with BM and hypertension are limited, the use of beta blockers and prognosis of patients with BM (20) reported that the survival rate improves relapse-free (95% CI, 0.10 to 0.87) and overall survival rate (95% CI, 0.12 to 1.00) in women with BM.
Epidemiological studies have suggested that exercise, weight loss, nutrition and lifestyle changes, are inversely related as RF for BM and recurrence (21). Practising exercise equals walking 30 minutes six days a week and eating 5 or eating more servings of fruits and vegetables to reduce mortality 46% BM (95% CI, 0.31 to 0.98), mainly in BM with ER +, no significant effect for BM hormone receptor-negative, but exercise had no effect on deaths from BM, but if after diagnosis reduces deaths by 34% (95% CI, 0.57 to 0.77) and recurrence in 24% (95% CI, .66 to .87). After diagnosis exercise only provide significant benefits in patients with BMI ≥25 kg/m2, and exercise after diagnosis of BM reduces deaths by 50% (95% CI, 0.34 to 0.74) for BM RE +, with a significant effect for ER-negative BM (22-25). The definition of physical activity or exercise varies little in the studies generally defined as moderate recreational activity or exercise forms the metabolic equivalent (MET) is the measured value of energy expenditure 1.2 kcal/kg/h, where energy expenditure is state of rest, is equal to 1 MET. Therefore, an activity level of 3 METs require energy expenditure equal to three times the resting state spending, the unit of measure is the amount emitted per square meter of skin, for example, sleep 0.8 MET, sitting relaxes 1 MET, 3.4 MET walking, running 9.5 MET, other examples of moderate physical activity include walking, jogging, running, cycling, swimming, tennis, gymnastics, aerobics and (22); women doing the equivalent of walking at an average rate of less than 3 MET-hours per week, the risk of BM is reduced 20% with 8.9 MET hours per week (95% CI, 0.60 to 1.06) and is more significant 50% reduction to 14.9 MET-hours per week (95% CI, 0.31 to 0.82), and 44% to 23.9 MET-hours per week (95% CI, 0.38 to 0.84) and 40% with 24 hours or more MET hours per week (95%, 0.40 to 0.89), however, found no significant effect for exercise, even for 9 or more MET hours per week, in CM with ER and progesterone receptor (PR) negative, but physical activity as an adjunct to treatment of BC is potentially beneficial and should be included into radiotherapy and Qt (22-25) because it reduces estrogen levels by reducing body fat or reduced androgens by increasing sex hormone binding globulin (SHBG), improves the resistance to insulin, blood glucose and dietary modifications are effective to reduce the recurrence and mortality BM (26,27); when reducing calories in fat to 15% without compromising nutrition and compared with the control group, recurrences are lower in the group with the lowest intake of fat (95% CI, 0.60 to 0.98), and is more important for women with hormone receptor-negative BM (95% CI, 0.37 to 0.91) compared with ER + BM (95%, from 0.63 to 1.14) (27). In women with early BM indication of a diet rich in vegetables, fruit and fiber, but low in fat does not affect mortality or decreased (95%, from 0.72 to 1.15), or the incidence of a second BM (95% CI, 0.80 to 1.14) during follow-up of 7.3 years, when, consumed ≥5 servings of fruits and vegetables and exercise equivalent to taking 30 minute walk, six days a week is associated with lower mortality (21).
Alcohol consumption affects the recurrence and mortality of women survivors BM. Consumption of three or four or more alcoholic drinks per week increased 35% (95% CI, 1.00 to 1.83) the risk of recurrence of BM and 51% (95% CI, 1.00 to 2.29) for death (28) BM with hormone receptor positive or negative.
Carcinogenesis breast cancer
Insulin is associated with obesity and risk of BM, and increases directly regulates cell proliferation of breast tissue and BM cells, in postmenopausal women with hyperinsulinemia increases the incidence and mortality from BM 2.22 (95% CI, 1.39 to 3.53) when compared with women with normal or low levels of insulin, (29,30) poor diet, inappropriate lifestyle and C-peptide concentrations (a marker of insulin secretion) (31) greater than 1 ng/mL, is associated with increased death risk and BM in 35% (95% CI, 1.02 to 1.87). Together, hyperglycemia and hyperinsulinemia are associated with poor prognosis in patients with BM. Patients with BM 32 and T2DM and elevated baseline levels of hemoglobin A1c (HbA1c) ≥7.0% (this test measures average blood glucose for the past 3 months to know the diabetes mellitus) these figures increase rate of all-cause mortality when compared with values of 1,8).
The actions of insulin are indirect through the reduced availability of SHBG, a glycoprotein that binds to sex hormones, testosterone and estradiol specifically inhibits the function of these hormones, and growth factors; increasing serum sex hormones such as androgens and estrogens not bound to SHBG-like growth factors (IGF), increased the risk of BM in pre-and postmenopausal women, it also inhibits the production of SHBG and increased blood levels of, insulin-like growth factor-1 (IGF-I), also known as somatomedin C, increased mitogenic activity. This link is compatible with 50% BM (32,33) overexpressing receptors IGF-I, IGF-I increases the proliferation and survival of cancer cell lines. There is a positive association between T2DM 2 and BM where insulin is involved in the development of BM and metformin treatment prevents the development of BM in patients with T2DM (8,34,35); T2DM control with long-acting insulin, increases the incidence of BM, especially when used on average 5.6 years (95% CI, 01.01 to 06.05) (36). In contrast, metformin inhibits proliferation and colony formation of cells BM (37), tumor growth and reduced the incidence of BM, through the following mechanisms: (I) Inhibition of growth in general, (II) reduction of serum insulin and (III) Reduce body weight (37).
Also demonstrated an antiapoptotic effect BCTN cell lines, but no effect on BM luminal A, B and (38) subtypes of HER-2neu. The use of metformin is associated with 38% lower incidence of BM receptor-positive, postmenopausal women with T2DM (8,35,39), no significant effects for receptor-negative BM.
Role of leptin in breast cancer
Leptin, also known as OB protein is a hormone produced primarily in adipocytes and other organs such as the hypothalamus, ovary and placenta, is a product of the obesity genes to be synthesized and secreted into the tissue adipose increased adiposity is associated with higher serum leptin levels (24,34,40). Leptin regulates food intake and metabolism through its actions on the hypothalamic arcuate nucleus. Resistance to leptin in obese individuals is similar to insulin resistance in diabetic patients and reported a positive association between the risk of BM and serum leptin levels with elevated mRNA expression in adipocytes of the tumor periphery .
At the molecular level, high leptin expression in mammary epithelial cells promotes carcinogenesis through mechanisms such as cell proliferation: increased peripheral aromatization by aromatase enzyme responsible for the biosynthesis of estrogens where they promote certain cancers and other diseases and aromatase inhibitors are often used to treat those diseases MAPK, the acronym of mitogen-activated protein kinases, or mitogen-activated protein kinase, signal transducer and activator of transcription 3, also known as STAT3, is a transcription factor encoded by the human STAT3 gene, STAT family members in the presence of cytokines and growth factors, are phosphorylated by an associated receptor tyrosine kinases, which form homo-or heterodimers, translocate to the nucleus where act as activators of transcription STAT3 to mediate the expression of various genes in response to certain stimuli cell in cellular processes such as cell proliferation and apoptosis, the cyclin family member D cyclins, cell cycle regulatory proteins, there are three types closely related cyclin D, cyclin D1, cyclin D2 and cyclin D3, these cyclins are expressed in most proliferating cells and regulate cell cycle progression. Angiogenesis physiological process that involves the formation of new blood vessels from preexisting vessels, is a normal process during development and body growth and wound healing, but is also critical in the malignant transformation of growth tumor, the vascular endothelial growth factor (VEGF) is a signaling protein involved in angiogenesis, apoptosis is programmed cell death, which is genetically regulated by the gene or protein 53 (p53) or tp53, also called the “guardian of the genome”, located on the short arm of chromosome 17 (17p13) and encodes a transcription factor nuclear 43.7 kDa which is essential to induce cell response to injury DNA, arresting the cell cycle in the event of mutation. The p53 gene is a tumor suppressor gene that plays a role in apoptosis and cell cycle control. Caspases are a group of proteins belonging to the group of cysteine proteases, characterized by having a cysteine residue that mediates the breakdown of other proteins, are essential mediators of apoptosis and programmed cell death (caspase-9), in the cell cycle regulation (p21) a gene located on chromosome 6 (6p21.2 location), which encodes an inhibitor of cyclin-dependent kinase, directly inhibits enzyme activity of the cyclin-CDK2 and cyclin-CDK4. The protein encoded by the gene called p21 CDKN1A, by its acronym cyclin-dependent kinase inhibitor 1A: inhibitor of cyclin dependent kinase 1A is a protein that functions to regulate the progression of S phase cell cycle expression p21 is regulated by the p53 tumor suppressor gene involved in cell survival. Kinases (Akt) are signal transduction pathways leading to cell transformation and tumor progression, all these events complexes act in the cellular process of carcinogenesis of BM (41).
The role of leptin is involved in physiological processes such as reproduction, immunity or angiogenesis; directly increases IGF-I receptor, the receptor of insulin-like growth factor 1 IGF1R abbreviated bound to its receptor IGF-1 (receiver tyrosine kinase), initiates intracellular signaling and is one of the most potent natural activators of signal transduction PKB, a stimulator of cell growth and proliferation and potent inhibitor of apoptosis or programmed cell death, and similarly, the IGF-I conversely increases leptin receptor activity through phosphorylation and initiates the proliferation and migration of cancer cells. The use of inhibitors of growth factor receptor (EGFR), which is the cellular receptor of the cell surface of the members of the family of epidermal growth factor (EGF family), a subfamily related tyrosine kinase receptors: EGFR (ErbB-1), HER2/c-neu (ErbB-2), Her 3 (ErbB-3) and Her 4 (ErbB-4). Mutations affecting the expression or activity of EGFR cause cancer. EGFR inhibitors, erlotinib and lapatinib, in the management of metastases after administration of leptin and reduced IGF-I cell migration and are therapeutic BM and 70% of overexpress EGFR BMTN (42). The gene BRCA-1 (breast cancer 1, “breast cancer 1”) is a tumor suppressor gene, which regulate the cell cycle and prevent the uncontrolled proliferation. BRCA-1 protein product of this gene is part of the detection system and repair of DNA damage. Variations of this gene are involved in some cancers, especially breast cancer. The BRCA1 gene is located on the long arm (q) of chromosome 17, in mammary epithelial cells, mutations in the BRCA-1 gene are associated with BCTN and EGFR inhibition with erlotinib in individuals who overexpress BRCA-1, prevents or delay the development of hormone receptor-negative BM but BM effect on ER +, neither was effective in reducing carcinogenesis BM after (42).
The relationship between leptin and BM is supported by studies in obese animals overexpressing transforming growth factor alpha (TGF-α) and leptin deficient animals developed no BM, while other animals heterozygous and homozygous BM developed in 50% and 67% of cases, respectively being useful for the treatment with the receptor antagonists of leptin as 92% of the leptin receptor expressing BM and 86% expressed leptin (43). Allo-ACA peptide antagonist, leptin receptor, increases to 80% survival rate in obese patients overexpressing leptin or the use of cetuximab, humanized IgG1 antibody with anti-EGFR ixabepilone, cisplatin, carboplatin, or taxanes, BM patients treated metastatic paclitaxel or docetaxel with weekly cetuximab (44), 82% showed decreased metastases, but 27% developed brain metastases during treatment.
The therapeutic value of EGFR inhibitors in combination with Qt including (I) carboplatin plus cetuximab and irinotecan (II) and carboplatin plus cetuximab; compared with carboplatin plus cetuximab cetuximab alone in patients with metastatic BCTN, 18% responded to treatment compared with 6% who received the cetuximab alone without improvement in objective response rate, disease-free and overall survival rate in all patients with metastatic BM when compared cetuximab with irinotecan and carboplatin compared with carboplatin, irinotecan more, objective response rate is higher in metastatic BM patients when using the three drugs (49%) compared with irinotecan alone more carboplatin (30%). These findings suggest a therapeutic benefit to the use of EGFR inhibitors in patients with BCTN (43,44).
The role of adiponectin in breast cancer
Adiponectin (also known as Acrp30, Adiponectin30, AdipoQ, apM1 or GBP28) a protein hormone is synthesized exclusively by adipose tissue that is involved in the metabolism of glucose and fatty acids, insulin sensitizer which is inversely correlated with obesity anti-oncogenic effects of leptin unlike that pro-carcinogenic effects. Adiponectin concentrations are reduced in obesity, T2DM and coronary artery disease.
Women with higher adiponectin concentrations are lower risk (65%) of BM (45). In women with BM on clinical stage I-IIIA and adiponectin levels greater than 15.5 mg/mL are associated with better survival rate (95% CI, 0.15 to 0.95) (30) and outcome in receptor-negative BM ineffective for women with hormone receptor-positive BM (=0.027) (46). Patients with low levels of adiponectin defined as the first percentile were more likely cancer recurrence compared with patients in the fourth percentile figures (95% CI, 1.03 to 7.68). Genetic factors are also associated with BM adiponectin. The role of adiponectin on risk of BM via a haplotype is a set of single nucleotide polymorphisms (SNPs) single nucleotide polymorphisms (SNPs), the haplotype tagging SNPs genotyped 10 of adiponectin (ADIPOQ) and the type I receptor gene adiponectin (AdipoR1), two functional polymorphisms of ADIPOQ and a functional polymorphism alters mRNA levels of AdipoR1, are significantly associated with increased risk of BM. When classified by signaling effect, low adiponectin signaling increases 6.56 times the risk of BM (95% CI, 0.78 to 54.89), and intermediate signaling effects of adiponectin increased the risk 4.16 times (95% CI, 0.49 to 35.19) compared with high effect signaling (bias =0.001), this indicates a significant role of adiponectin in predicting risk of CM, via signaling mechanisms that are involved in BM carcinogenesis, specifically, a number of compounds related to cell proliferation (aromatase, MAPK, and cyclin D1), apoptosis (Bcl-2 and caspase 8), are key mediators of apoptosis and programmed cell death. Caspase cascade can be activated by granzyme B released by cytotoxic T lymphocytes (CD8+) and active caspase-3 and caspase-7, for cell death receptors such as Fas, TRAIL or TNF to activate caspase-8 and caspase-10 or the apoptosome, regulated by the Bcl-2 and cytochrome c that active caspase-9. Once activated, this cascading process positive feedback ensures that the cell will inevitably suffer apoptosis by regulating the cell cycle (AMPK) and cell survival (Akt) which are involved in the carcinogenesis of BM (41). Adiponectin has antiproliferative effects on the growth of cell lines BM hormone receptor positive and negative, but by different mechanisms (45), presumably through induction of apoptosis (41) as the major division of the poly (ADP-ribose) polymerase (PARP), which are biomarkers for early apoptosis was detected in cell lines with RE + BM also reported that adiponectin inhibits aromatase activity and estrogen receptor mechanisms involved in ER + BM (41). These data suggest that adiponectin acts through multiple signaling pathways with different predominant mechanisms BM cell lines with hormone receptor positive or negative.
Overexpression of adiponectin, both locally and systemically, reduces the size of the BM. In contrast, reduction of adiponectin overexpression accelerates development and tumor progression and associated proposed mechanisms that low levels of adiponectin and mammary carcinogenesis are:
(I) interaction with insulin (40);
(II) Interaction with leptin (35);
(III) inhibition of tumor necrosis factor alpha (TNF-α), is a protein from the group of cytokines that are released from cells of the immune system in macrophages (47);
(IV) binding to fibroblast growth factor (FGF, for its acronym in English (fibroblast growth factor) is a growth factor that increases the rate of mitotic activity and DNA synthesis by facilitating the proliferation of various stem cells, as chondroblast, colagenoblasto, osteoblast, etc, related to tumor angiogenesis in malignant neoplasms and a polypeptide growth factor beta, platelet-derived (PDGF, for its acronym in English platelet derived growth factor) is one of many growth factors, or proteins that regulate cell growth and cell division particularly for angiogenesis, which involves the growth of blood vessels from existing vascular tissue, where uncontrolled angiogenesis is characteristic of cancer (48);
(V) inhibition of nuclear factor NF-κB modulates the differentiation of mesenchymal cell lines, via post-transcriptional inhibition of key developmental regulators.
Thus, nuclear factor kappa B (NF-κB) is a transcription factor vital cellular, controls and regulates the expression of various genes that are important in the inflammatory and immune processes, proliferation and cell death, viral replication, production and nitric oxide controls cell interaction mediated myogenesis suppressing TNF-alpha through a mechanism causing MyoD mRNA inhibition NF-κB that stimulates the expression of genes encoding cytokines e.g., TNF-alpha, interleukins are a number of cytokines (proteins that act as chemical messengers that are synthesized primarily from leukocytes, but may be involved in endothelial cells, the main function is to regulate the functions of immune system cells, such as activation, differentiation or proliferation, antibody secretion, chemotaxis, regulation of other cytokines and other factors and are IL-1, IL-6, IL-2, IL-12. interferon gamma (IFN-γ), also called immune interferon or type II, is a type of cytokine produced by T lymphocytes and natural killer (NK) whose main function is activation of macrophages, immune responses in both innate and adaptive cellular responses. stimulating factor granulocyte-monocyte colony or GM-CSF in English (Granulocyte Macrophage Colony-Stimulating Factor), is a family of glycoproteins that modulate hematopoiesis and survival control, proliferation, differentiation and functional capacity of hematopoietic progenitors with activities often overlapping. are important regulators of the immune response and tissue homeostasis. cellular adhesion molecules (e.g., selectins are adhesion receptors that form hetero-and homotypic junctions, transient and specific, we have identified three members of this family, which correspond to leukocyte differentiation antigens CD62L (L-selectin), CD62P (P-selectin) and CD62E (E-selectin), these three molecules recognize and bind, via its lectin domain are sugar binding proteins with high specificity for each different type of various oligosaccharides, which are usually transmembrane proteins conjugated. E-selectin (CD62E) is not expressed de novo in endothelial cells, as a result of induction of expression of the corresponding gene during cell activation. Molecular Cell Adhesion The (CAMs) are glycoproteins located on the cell surface which constitutes cell receptors are also found in the tissue matrix and made specific cell-cell interactions and cell-matrix, the adhesion molecules expressed in activated vascular endothelial cells, macrophages, dendritic cells and bone marrow stroma are VCAM-1, and ICAM-1 laa expressing lymphocytes, macrophages, activated endothelial cells and epithelial cells. chemokines (also called chemokines) are small proteins belong to a family of cytokines. are called in this way due to the ability to induce chemotaxis in the vicinity of responsive cells, some chemokines are chemotactic cytokines play a role in development: promote angiogenesis (new blood vessel growth) or guide cells into tissues that provide specific critical signals for cell maturation (e.g., IL-8, MIP-1 for short macrophage inflammatory protein MIP-1alpha, MCP-1 for its acronym in English methyl-accepting chemotaxis protein 1, CCL5 or RANTES that attract cells such as T cells, eosinophils and basophils and eotaxin and chemokine receptors, e.g., major histocompatibility complex (MHC for its acronym in English major histocompatibility complex), or major histocompatibility complex, is a family of genes located on the short arm of chromosome 6 whose products are involved in antigen presentation to T lymphocytes and inducible enzymes (for example inhibitors of cyclooxygenase-2 (COX-2), also called coxibs, are anti-inflammatory steroidal nitric oxide synthase enzyme responsible for transforming the L-arginine into nitric oxide there are three known isoforms, two are constitutive (cNOS) and the third is inducible (iNOS) it protects the immune system against pathogens and share antimicrobial and antitumor activities as part of the oxidative burst of macrophages. p105 subunit of nuclear factor NF-kappa-B (NFKB1) is a human protein encoded by the gene NFKB1 is a transcription factor that is activated by a variety of intra-and extracellular stimuli, such as cytokines, reactive oxygen species, ultraviolet (UV) and bacterial and viral products. inappropriate activation of NF-κB is associated with a number of inflammatory diseases, while a persistent inhibition of NF-κB leads to an improper development of immune cells or cellular growth delay also increases the expression of important molecules in the regulation of cell proliferation, apoptosis and cell cycle progression, such as IAPs by its English acronym cellular inhibitor of apoptosis protein or apoptosis inhibitory proteins there are five different XIAP (X-linked inhibitor of apoptosis), c-IAP1, c-IAP2, and survivin neuronal IAP. All possess anti-apoptotic activity in vitro. apoptotic stixmuli spectrum they are blocked by apoptosis inhibitory protein or cellular inhibitor of apoptosis protein IAPs is broad and includes transducers ligands and receptor family of receptor-associated factor or TNF-TNF receptor-associated factor (TRAF) 1, TRAF2 (TNF), pro-apoptotic members of the Bcl-2 family, Cytochrome c and chemotherapeutic agents. XIAP has wide and large capacity antiapoptotic. XIAP, c-IAP1 and c-IAP2 are direct inhibitors of caspases. They bind to and inhibit caspases -3 and -7 also active and procaspase-9, but not caspase-1, -6, -8 or -10. NF-κB also increases the expression of important molecules in the regulation of cell proliferation, apoptosis and cell cycle progression, such as c-IAP) 1, c-IAP2, TRAF 1, TRAF2, Bcl-2 (B-cell lymphocyte/leukemia-2) homologs (AF1/BFI-1, IEX -IL), Fas, c-myc and cyclin D1;
(VI) Promotion of angiogenesis.
Conclusions
Studies of carcinogenesis and their association BM MS, central obesity, insulin resistance, glucose intolerance, dyslipidemia, and hypertension and carcinogenesis of different subtypes of BM are limited but there are reports that provide this association tests abdominal obesity and increased weight defined, increases the incidence of BCTN. Moreover, T2DM and insulin resistance are associated with increased incidence of BM, BM progression in women with obesity worse prognosis and higher recurrence in all subtypes of CM (16) similarly hyperglycemia and hyperinsulinemia are associated with increased incidence and poor prognosis (29,30,31,49), changes in lifestyle, moderate exercise, diet rich in fruits, vegetables, and micronutrients and lower alcohol consumption in combination with Qt improve the survival rate in BM.
Molecular mechanisms have been proposed and different components of the metabolic syndrome in the modulation of carcinogenesis and progression of BM,. inflammatory markers such as cytokines (such as TNF, IL-1, IL-6, and chemokines), enzymes (such as COX2, 5-LOX, MMP-9), and adhesion molecules (ICAM-1, ELAM- 1 and VCAM-1) are closely associated with carcinogenesis, and such substances have been shown to be regulated by nuclear transcription factor, NF-κB, and this has been shown to control expression of this linked gene products, such as cell survival tumor or anti-apoptosis (Bcl-2, Bcl-XL, PAI-1, PAI-2, XIAP, survivin, cFLIP, and TRAF-1), proliferation (such as c-myc and cyclin D1), invasion (MMP-9) and angiogenesis (VEGF). Insulin is involved in the risk of BM through direct and indirect effects, resulting in a higher concentration of (32,33) androgens, estrogens, and increased IGF-I. Leptin and adiponectin, which are secreted by adipose tissue and BM, act through low signaling pathways involved in cell proliferation, apoptosis, cell cycle regulation, cell survival and angiogenesis (41). Normal cells maintain a delicate balance between leptin and adiponectin in order to maintain cellular homeostasis in the tissue, but the components of the metabolic syndrome disturb this balance by increasing serum levels of leptin and adiponectin reduction; insulin IGF-I and EGFR plays a role in mediating interactions between these two hormones from (42,50-53), see Figure 1.
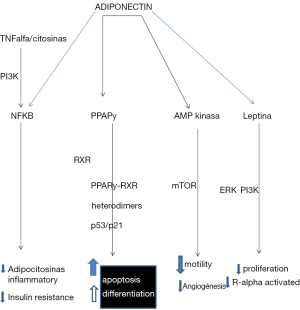
The components of metabolic syndrome and insulin-axis-adiponectin leptin play a key role in the pathogenesis and progression of TNBC. At present, treatment for this form of cancer are limited compared to other subtypes of MC, because these tumors are resistant to treatments targeting hormonal therapy and-white against HER-2neu although Qt trastuzumab, anti -EGFR, anti-angiogenic drugs and inhibitors of PARP are favorable, the change in life style, reduce the risk of recurrence of BCTN and metformin or antagonists of leptin, as well as other therapies which modify insulin-axis leptin -adiponectin are useful in the prevention and treatment of BCTN (7,8,50,51).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Sasa M, Bando Y, Takahashi M, et al. Screening for basal marker expression is necessary for decision of therapeutic strategy for triple-negative breast cancer. J Surg Oncol 2008;97:30-4. [PubMed]
- Martinez E, Magro A, Chirivella I, et al. Cáncer mamario basal-like. In: Vargas-Hernández VM. eds. Cáncer en la Mujer, Edit. Alfil. México 2011:1907-19.
- Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 2008;14:1368-76. [PubMed]
- Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 2007;109:1721-8. [PubMed]
- Morris GJ, Naidu S, Topham AK, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer 2007;110:876-84. [PubMed]
- Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492-502. [PubMed]
- Quirós AJL, Jimenez RY, Jimenez ME, et al. Carcinomas invasores triples negativosde la glándula mamaria: incidencia y características clínico-patológicas. Acta Med Costarric 2010;52:90-5.
- Currie CJ, Poole CD, Jenkins-Jones S, et al. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012;35:299-304. [PubMed]
- Brenton JD, Carey LA, Ahmed AA, et al. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol 2005;23:7350-60. [PubMed]
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. [PubMed]
- Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev 2003;4:157-73. [PubMed]
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007;121:856-62. [PubMed]
- Maiti B, Kundranda MN, Spiro TP, et al. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat 2010;121:479-83. [PubMed]
- Kitahara CM, Berrington de González A, Freedman ND, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol 2011;29:1592-8. [PubMed]
- Peeters PH, van Noord PA, Hoes AW, et al. Hypertension and breast cancer risk in a 19-year follow-up study (the DOM cohort). Diagnostic investigation into mammarian cancer. J Hypertens 2000;18:249-54. [PubMed]
- Vargas-Hernández VM, Vargas AVM, Acosta AG, et al. Estudio y Manejo de la obesidad en mujeres y su riesgo oncológico. In: Vargas-Hernández VM. eds. Cáncer en la Mujer, Edit. Alfil. México, 2011:1907-19.
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [PubMed]
- Ewertz M, Jensen MB, Gunnarsdóttir KÁ, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol 2011;29:25-31. [PubMed]
- Ademuyiwa FO, Groman A, O’Connor T, et al. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer 2011;117:4132-40. [PubMed]
- Litton JK, Gonzalez-Angulo AM, Warneke CL, et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol 2008;26:4072-7. [PubMed]
- Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 2011;29:2645-52. [PubMed]
- Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol 2007;25:2345-51. [PubMed]
- Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA 2005;293:2479-86. [PubMed]
- Abrahamson PE, Gammon MD, Lund MJ, et al. Recreational physical activity and survival among young women with breast cancer. Cancer 2006;107:1777-85. [PubMed]
- Irwin ML, Smith AW, McTiernan A, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol 2008;26:3958-64. [PubMed]
- Enger SM, Bernstein L. Exercise activity, body size and premenopausal breast cancer survival. Br J Cancer 2004;90:2138-41. [PubMed]
- Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst 2006;98:1767-76. [PubMed]
- Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 2007;298:289-98. [PubMed]
- Kwan ML, Kushi LH, Weltzien E, et al. Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: the life after cancer epidemiology study. J Clin Oncol 2010;28:4410-6. [PubMed]
- Kabat GC, Kim M, Caan BJ, et al. Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. Int J Cancer 2009;125:2704-10. [PubMed]
- Duggan C, Irwin ML, Xiao L, et al. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol 2011;29:32-9. [PubMed]
- Erickson K, Patterson RE, Flatt SW, et al. Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol 2011;29:54-60. [PubMed]
- Shimizu C, Hasegawa T, Tani Y, et al. Expression of insulin-like growth factor 1 receptor in primary breast cancer: immunohistochemical analysis. Hum Pathol 2004;35:1537-42. [PubMed]
- Davison Z, de Blacquière GE, Westley BR, et al. Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: implications for therapy. Neoplasia 2011;13:504-15. [PubMed]
- Gonzalez-Chavez A. Diabetes y cáncer. In: Vargas-Hernández VM. eds. Cáncer en la Mujer, Edit. Alfil. México, 2011:857-62.
- Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304-5. [PubMed]
- Suissa S, Azoulay L, Dell’Aniello S, et al. Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia 2011;54:2254-62. [PubMed]
- Liu B, Fan Z, Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle 2009;8:2031-40. [PubMed]
- Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 2009;8:909-15. [PubMed]
- Chlebowksi RT, McTiernan A, Fan Z, et al. Metformin and breast cancer incidence in postmenopausal diabetic women in the Women’s Health Initiative (WHI). J Clin Oncol 2011;29:abstr 1503.
- Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertil Steril 2002;77:433-44. [PubMed]
- Jardé T, Perrier S, Vasson MP, et al. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer 2011;47:33-43. [PubMed]
- Burga LN, Hu H, Juvekar A, et al. Loss of BRCA1 leads to an increase in epidermal growth factor receptor expression in mammary epithelial cells, and epidermal growth factor receptor inhibition prevents estrogen receptor-negative cancers in BRCA1-mutant mice. Breast Cancer Res 2011;13:R30. [PubMed]
- Otvos L Jr, Kovalszky I, Riolfi M, et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer 2011;47:1578-84. [PubMed]
- Nechushtan H, Steinberg H, Peretz T. Preliminary results of a phaseI/II of a combination of cetuximab and taxane for triple negative breast cancer patients. J Clin Onco 2009;27:e12018.
- Körner A, Pazaitou-Panayiotou K, Kelesidis T, et al. Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J Clin Endocrinol Metab 2007;92:1041-8. [PubMed]
- Oh SW, Park CY, Lee ES, et al. Adipokines, insulin resistance, metabolic syndrome, and breast cancer recurrence: a cohort study. Breast Cancer Res 2011;13:R34. [PubMed]
- Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res 2005;96:838-46. [PubMed]
- Irwin ML, Duggan C, Wang CY, et al. Fasting C-peptide levels and death resulting from all causes and breast cancer: the health, eating, activity, and lifestyle study. J Clin Oncol 2011;29:47-53. [PubMed]
- Van der Veldt AA, Lubberink M, Bahce I, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell 2012;21:82-91. [PubMed]
- Macis D, Gandini S, Guerrieri-Gonzaga A, et al. Prognostic effect of circulating adiponectin in a randomized 2 x 2 trial of low-dose tamoxifen and fenretinide in premenopausal women at risk for breast cancer. J Clin Oncol 2012;30:151-7. [PubMed]
- Fabian CJ. Adiponectin: a risk biomarker and attractive target for chemoprevention. J Clin Oncol 2012;30:124-6. [PubMed]
- Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol 2012;30:164-71. [PubMed]


