Meta analysis of efficacy and safety between Mammotome vacuum-assisted breast biopsy and open excision for benign breast tumor
Along with increased awareness programs, more breast lesions have been detected by ultrasound and many other examinations. Most of them are benign. The conventional surgical resection often has large incision(s) and will leave permanent scar(s) on breast skin, and thus can not meet the patients’ demand for minimizing the cosmetic damage. Therefore, Mammotome vacuum-assisted breast biopsy (VABB) systems have gradually been applied for treating benign breast lesions. The Mammotome VABB systems were initially employed for the biopsy of breast lesions; since its rotation knife can continuously cut the breast lesion and thus completely remove the small lesions (1), it can serve as a minimally invasive operation. As shown in some studies, the clinically non-palpable small breast lesions can be satisfactorily treated with Mammotome VABB (2) and therefore is a main indication of this procedure (3). Currently, while many studies have explored the role of Mammotome VABB for benign breast lesions, few relevant Meta analyses have been conducted in this era of evidence-based medicine. In our currently, by conducting Meta analysis on 5,256 cases from 15 articles using the most updated theories of modern evidence-based medicine, we tried to compare the effectiveness and safety of Mammotome VABB with those of conventional surgeries.
Subjects and methods
Inclusion and exclusion criteria
Research types
All the randomized controlled trials (RCTs) or clinical controlled trials (CCTs) pertaining to the Mammotome VABB and open surgery for benign breast tumors that have been published in Chinese and English journals were enrolled in this analysis. The CCTs included those with incomplete or incorrect randomization methods, no matter allocation concealment or blinding was employed. The inclusion of studies was not limited by sample size or follow-up duration.
Subjects
Inclusion criteria: (I) The removed breast tumor was pathologically confirmed to be benign, with the tumor size <3 cm; and (II) the whole surgical procedure was completed under the real-time monitoring of ultrasound. The identified articles were read carefully, and relevant variables were extracted from each study. For the missing data, we contacted the corresponding authors to supplement them.
Exclusion criteria: (I) duplicate publications; (II) publications with few information or incomplete data or being inaccessible; (III) reviews, case reports, and conference reports; (IV) original articles without control group (treated with open surgeries); (V) articles in which the control group was not treated with conventional surgery; rather, it was treated with other procedures such as endoscopy, EnCor (another minimally invasive system), or advanced breast biopsy instrumentation (ABBI); (VI) literature assessing the diagnostic value of Mammotome VABB for benign breast lesions; (VII) literature assessing the therapeutic value of Mammotome VABB for malignant breast tumors; (VIII) literature assessing the diagnostic and therapeutic values of Mammotome VABB for breast calcification, gynecomastia, and some other diseases; and (IX) literature reporting the use of Mammotome VABB under MRI or mammography.
Interventions
The Mammotome group included patients who had received Mammotome VABB for benign breast lesions, and the control group received convention open excision.
Measures
Measures were divided into primary and secondary measures. The primary measures included tumor size, incision length, intraoperative blood loss, operative time, recovery time, and scar size. The secondary measures included postoperative bleeding, wound infection, subcutaneous ecchymosis, hematoma, residual tumor, and breast deformation.
Literature search
Search method
Relevant studies were selected by searching Medline, PubMed, Embase, Ovid, Cochrane Library, VIP, Wanfang, CNKI and Chinese Biological Medicine Database, and some literature was manually searched. Literature of any language was searched. All the publications including conference papers and dissertations were searched.
Search strategies
Computer retrieval was conducted in accordance with the search strategies developed by the Cochrane Collaboration. English literature was searched using the following search terms: “vacuum-assisted breast biopsy/benign”, “Mammotome”, and “surgery/operation/minimally invasive”. The same terms in Chinese were used for searching Chinese literature. Meanwhile, free text word and MeSH terms were also used. All the literature was published before January 1, 2012. A total of 1,050 articles were retrieved, and the references and other publications were reviewed to identify all the relevant randomized clinical research.
Research methods
Literature screening and data extraction
Literature screening, data extraction, and cross-check were independently conducted by two reviewers to ensure the concordance of extracted data. The titles and abstracts of these articles were read firstly; for those whose data could not be easily judged, the full-text articles were downloaded and read. Literature was strictly screened according to the inclusion/exclusion criteria. Any disagreement between these two reviewers was resolved by discussion or by consulting a third expert. If the relevant data were insufficient, the corresponding authors of the original articles were contacted to supplement them. Inaccessible data were not further handled.
Evaluation of literature quality
The risk of bias was evaluated according to the quality evaluation standards in the Cochrane Manual (http://www.cochrane-handbook.org/) (4).
Statistical analysis
The Cochrane Collaboration’s RevMan 5.0 software was used for data analysis. The merging of data was based on the results of heterogeneity tests: If there was no statistically significant between-study heterogeneity (I2<50%), data were merged using a fixed effect model, and the OR value and its 95% confidence intervals (CI), Z value, and probability were calculated. If there was statistically significant between-study heterogeneity (I2>50%), data were merged using a fixed effect model for studies with homogeneity, or, data were merged using a random effect model and then underwent sensitivity analysis.
The enumeration data (mainly the outcome measures) are presented as odds ratio (OR) and 95% CI. The measurement data, particularly those obtained from the measurement using the same scale in different clinical trials, were presented as mean different (MD) and 95% CI. If Meta analysis showed that there was significant difference between these two surgical procedures, tunnel plots were used to analyze the likely presence of bias.
Results
Results of literature search and evaluation of methodological quality
A total of 1,050 articles were retrieved. After reading the titles and abstracts, the researchers found 19 relevant articles. After the two reviewers carefully read the full texts, two reviews and two non-randomized trials were excluded. Totally 15 articles (5-19) (n=5,256) entered the final analysis. According to the literature quality evaluation criteria, one article was rated as grade B and 14 as grade C (Tables 1,2).
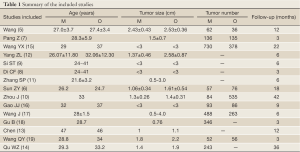
Full Table
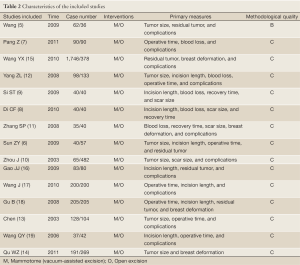
Full Table
Results of meta analysis
Primary measures
(I) Tumor size
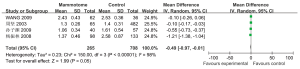
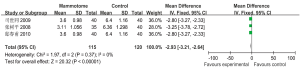
Four studies reported the tumor sizes in two groups. According to the results of heterogeneity test, there was statistically significant heterogeneity among the studies (P2=98%), and a random effect model was applied for Meta analysis, which showed that the tumor size was not significantly different between these two groups [MD=–0.49, 95% CI (–0.97, –0.01), P=0.05] (Figure 1).
(II) Incision length
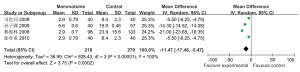
Four studies reported the incision length in two groups, which showed statistically significant heterogeneity (P2=100%). A random effects model was applied for Meta analysis, which showed that the incision length was significantly shorter in the Mammotome group than in the control group [MD=–11.47, 95% CI (–17.48, –5.47), P=0.0002] (Figure 2).
(III) Intraoperative blood loss
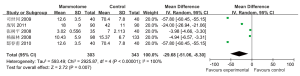
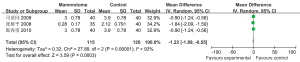
Five studies reported the intraoperative blood loss in two groups, which showed statistically significant heterogeneity (P2=100%). A random effects model was applied for Meta analysis, which showed that the intraoperative blood loss was significantly fewer in the Mammotome group than in the control group [MD=–29.68, 95% CI (–51.06, –8.30), P=0.007] (Figure 3).
(IV) Operative time
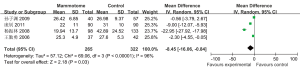
Four studies reported the operative time in two groups, which showed statistically significant heterogeneity (P2=96%). A random effects model was applied for Meta analysis, which showed that the operative time was significantly shorter in the Mammotome group than in the control group [MD=–8.45, 95% CI (–16.06, –0.84), P=0.03] (Figure 4).
(V) Recovery time
Three studies reported the recovery time in two groups, which showed no statistically significant heterogeneity (P2=0%). A fixed effects model was applied for Meta analysis, which showed that the recovery time was significantly shorter in the Mammotome group than in the control group [MD=–2.93, 95% CI (–3.21, –2.64), P=0.001] (Figure 5).
(VI) Scar size
Three studies reported the scar size in two groups, which showed statistically significant heterogeneity (P2=93%). A random effects model was applied for Meta analysis, which showed that the scar size was significantly smaller in the Mammotome group than in the control group [MD=–1.22, 95% CI (–1.89, –0.55), P=0.0003] (Figure 6).
Secondary measures
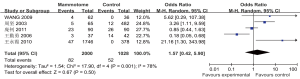
(I) Postoperative blood loss
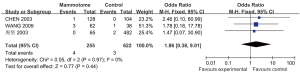
Three studies reported the incidence of postoperative blood loss in two groups, which showed no statistically significant heterogeneity (P=0.97, I2=0%). A fixed effects model was applied for Meta analysis, which showed that the incidence of postoperative blood loss was not significantly different between these two groups [OR=1.86, 95% CI (0.38, 9.01), P=0.44] (Figure 7).
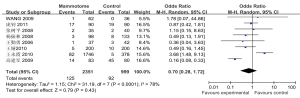
(II) Wound infections
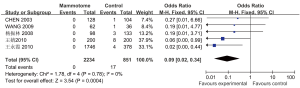
Five studies reported the incidence of postoperative wound infections in two groups, which showed statistically significant heterogeneity (P2=0%). A fixed effects model was applied for Meta analysis, which showed that the incidence of postoperative wound infections was significantly lower in the Mammotome group than in the control group [OR=0.09, 95% CI (0.02, 0.34), P=0.0004] (Figure 8).
(III) Subcutaneous ecchymosis
Five studies reported the incidence of subcutaneous ecchymosis in two groups, which showed statistically significant heterogeneity (P=0.001, I2=78%). A random effects model was applied for Meta analysis, which showed that the incidence of subcutaneous ecchymosis was not significantly different between these two groups [OR=1.57, 95% CI (0.42, 5.90), P=0.50] (Figure 9).
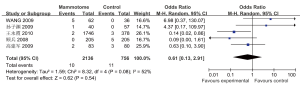
(IV) Hematoma
Eight studies reported the incidence of hematoma in two groups, which showed statistically significant heterogeneity (P2=78%). A random effects model was applied for Meta analysis, which showed that the incidence of postoperative hematoma was not significantly different between these two groups [OR=0.70, 95% CI (0.28, 1.72), P=0.43] (Figure 10).
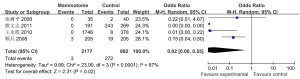
(V) Residual tumor
Five studies reported the incidence of residual tumor in two groups, which showed statistically significant heterogeneity (P=0.08, I2=51%). A random effects model was applied for Meta analysis, which showed that the incidence of residual tumor was not significantly different between these two groups [OR=0.61, 95% CI (0.13, 2.91), P=0.54] (Figure 11).
(VI) Breast deformation
Four studies reported the incidence of breast deformation in two groups, which showed statistically significant heterogeneity (P2=87%). A random effects model was applied for Meta analysis, which showed that the incidence of breast deformation was significantly lower in the Mammotome group than in the control group [OR=0.02, 95% CI (0.00, 0.55), P=0.02] (Figure 12).
Sensitivity analysis
When the fixed effects models were applied, the overall effect MD (95% CI) of the incision length, intraoperative blood loss, operative time, and postoperative scar size were –13.06 (–13.26, –12.85), –10.11 (–10.70, –9.53), –5.43 (–6.94, –3.93), and –1.35 (–1.53, –1.18) (Z=125.73, 33.97, 7.06, and 15.2) (all P<0.001). The overall effect OR (95% CI) of postoperative subcutaneous ecchymosis, hematoma, residual tumor, and breast deformation between these two groups were 1.33 (0.86, 2.07), 0.73 (0.53, 1.01), 0.62 (0.27, 1.40), and 0.01 (0.01, 0.03) (Z=1.29, 1.91, 1.16, and 10.42; P=0.20, 0.06, 0.25,<0.001, respectively). Except for the tumor size, the results were consistent between the random and fixed effects models, suggesting that the findings of this study were basically reliable.
Publication bias
Funnel plots were constructed for all the measures using RevMan 5.0 software. The funnel plots were basically symmetrical, indicating that there was no significant publication bias.
Discussion
The relatively low quality of these 15 studies affects the power of this Meta analysis. Due to the limitations of the included publications, the descriptions of measures used in each study were not consistent. The parameters in one or two studies were converted in our analysis, and therefore the stability of the results might be impaired. Although the results must be cautiously interpreted, they are still informative for clinical practices.
In the past two decades, the rapid development of minimally invasive techniques has facilitated the increasingly wide application of minimally invasive surgeries in clinical settings. Due to the high requests for cosmesis, minimally invasive techniques have also been commonly applied in the department of breast surgery. The introduction of Mammotome VABB systems is a milestone event in the application of minimally invasive techniques in breast surgery.
The Mammotome VABB system, firstly reported by Burbank et al. in 1994, was initially developed for the biopsy of breast diseases. However, along with the development of the relevant techniques, it has gradually been applied for the removal of various breast lesions. It has high diagnostic accuracy and is featured by high efficiency and low invasiveness when applied for surgical resection. Up to now, many countries have introduced this technique (20). Fine et al. (21) evaluated 124 women with low-risk palpable lesions. Lesions 1.5 cm but et al. (22) performed ultrasound-guided vacuum-assisted biopsy in 25 cases, with guidance using the 11-gauge probe. Complete excision was achieved in all lesions less than or equal to 1.5 cm. Of the 20 lesions measuring 1.5 to 2.0 cm, 11 (55%) were completely excised. They concluded that Mammotome VABB may provide an option for the definitive treatment of breast fibroadenomas. Plantade et al. (23) evaluated the use of Mammotome VABB for diagnosis and treatment of probably benign breast masses. Under the guidance of ultrasound, 79.1% of the benign lesions were completely removed, and the complication rate was 1.3%. It was therefore believed that the ultrasound-guided Mammotome VABB was an alternative to surgery for benign breast masses less than 15 mm.
In our current Meta analysis, the findings of 15 articles were pooled, and their merged effects were calculated. Compared with the conventional surgery, the Mammotome VABB had smaller surgical incision, less intraoperative blood loss, shorter operative time, shorter recovery time, and smaller scar size; furthermore, for benign breast lesions less than 3 cm, both groups showed no significant difference in terms of tumor size. Therefore, for small benign breast tumors, Mammotome VABB is actually a minimally invasive surgery. More specifically, the conventional open surgery has several disadvantages: (I) the surgery often leaves a large incision on the breast surface; (II) the large surgical wound often results in massive intraoperative blood loss; (III) for deep or clinical palpable small lesions, the open surgery can be time-consuming, and sometimes extended resection may be needed, which can cause further damage (10); and (IV) accordingly, the recovery time is long, and the scar size is large. In contrast, the Mammotome VABB requires small incision; under the guidance of high-frequency color ultrasound, the intraoperative positioning will not be limited by the lesion site, breast size, and gland density. Thus, since the tiny foci become visible, the Mammotome VABB can minimize the blindness of surgery and thus reduces the surgical wound, decreases blood loss, and shortens operative time. After the surgery only a 0.3-cm scar will be left on the breast skin, and therefore the cosmetic effectiveness is satisfactory.
The postoperative bleeding, percutaneous ecchymosis, and hematoma are all related with bleeding. Although Mammotome VABB can not perform suture to stop bleeding (as done in open surgery), it is performed under the guidance of ultrasound, which can display the tiny lesions and vessels and thus allows the operators to reduce intraoperative blood loss by avoiding blood vessels during puncture (9). As surgical experience accumulates, most Mammotome procedures have adopted corresponding measures to prevent the post-operative bleeding. These measures include: adding adrenaline to preoperative anesthetic solution; aspiration of residual blood after the mass is removed; local hemostasis for 15 min after surgery; and use of elastic bandages around the chest after surgery (10). After the application of the above measures, the post-operative incidences of bleeding, subcutaneous bruise/ecchymosis, and hematoma have been not significantly different between the Mammotome group and the open surgery group.
In the earlier cases, the Mammotome procedures were associated with residual tumors. However, as experiences accumulate, only benign tumors less than 3 cm were selected for Mammotome VABB, which ensures the complete resection of the breast lesions. Thus, for these tumors, the incidence of residual tumor has shown no significant difference between Mammotome VABB and conventional open surgery.
The incidences of postoperative wound infections and breast deformation are higher in the open surgery group than in the Mammotome group. As mentioned above, the open surgery can result in larger wound, longer operative time, and more blood loss; accordingly, the wound infection rate can be high. Furthermore, to ensure the accurate resection of the lesions, relatively more normal breast tissues will be removed, resulting in gland defects and poor cosmetic outcomes (e.g., breast collapse and deformation). In contrast, Mammotome VBAA enables the accurate and rapid removal of lesions under the real-time monitoring of ultrasound. It has shorter operative time and less blood loss. Notably, since it need not remove many normal breast tissues, it will not result in gland defect, and therefore the wound infection rate is low and the breast shape remains acceptable (10).
In summary, the Mammotome VABB reflects the unique concept and advantages of minimally invasive surgery. It can effectively reduce the surgical incision, decrease blood loss, shorten operative time and recovery duration, shrink surgery scars, and maintain good post-operative appearance, which is helpful to decrease the physiological and psychological trauma to the patients and represents a future trend of “minimally invasive” surgery. It will play a key role in treating benign breast diseases. However, limited by the length of the rotation knife, it is not feasible for mass sized 3 cm or larger (24). Nevertheless, comparison between Mammotome VABB and conventional surgery still provide objective evidence-based evidence for the clinical treatment of benign breast tumors.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Fine RE, Israel PZ, Walker LC, et al. A prospective study of the removal rate of imaged breast lesion by an 11G gauge vacuum-assisted biopsy probe system. Am J Surg 2001;182:335-40. [PubMed]
- Chun K, Velanovich V. Patient perceived cosmesis and satisfaction after breast biopsy: comparison of stereotactic incision, excisional, and wire localized biopsy techniques. Surgery 2002;131:497-501. [PubMed]
- Cassano E, Urban LA, Pizzamiglio M, et al. Ultrasound-guided vacuum-assisted core breast biopsy: experience with 406 cases. Breast Cancer Res Treat 2007;102:103-10. [PubMed]
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5. 0. 1 [updated September 2008]. Hoboken: John Wiley & Sons Ltd., 2008: 193-4.
- Wang WJ, Wang Q, Cai QP, et al. Ultrasonographically guided vacuum-assisted excision for multiple breast masses: non-randomized comparison with conventional open excision. J Surg Oncol 2009;100:675-80. [PubMed]
- Sun Z, Song A, Sun Y, et al. Ultrasound guided mammotome system in excision of breast fibroadenomas. Chinese Journal of integrated traditional Chinese and Western Medicine 2009;15:581-3.
- Pang Z, Wang N. The clinical research of applying mammotome technique to treat breast tumor. Guangzhou: Jinan University 2011:1-23.
- Di C, Li Z, Wang H. Experience of minimally invasive operation and traditional operation for benign breast tumor. Guide of China Medicine 2010;8:301-2.
- Si S. Comparative study of minimally invasive operation and traditional operation of breast. Chinese Health and Nutrition. Clinical Journal 2009;18:96-8.
- Zhou J, Wang H, Zou Q, et al. Minimally invasive operation for benign breast lesion. Journal of Surgical Concepts and Practice 2003;8:314-6.
- Zhang S, Zou Y, Deng S, et al. Analysis of 75 patients with breast benign tumor ectomized by mini-cut mammotome or by opening operation. China Modern Doctor 2008;46:18-20.
- Yang Z, Cui G, Wang X, et al. Surgery selection of breast benign tumor excision. Chinese Archives of General Surgery: Electronic Version 2008;2:32-4.
- Chen SC, Yang HR, Hwang TL, et al. Intraoperative ultrasonographically guided excisional biopsy or vacuum-assisted core needle biopsy for nonpalpable breast lesions. Ann Surg 2003;238:738-42. [PubMed]
- Qu W, Wang M, Tu W, et al. Comparative study of minimally invasive surgery and traditional surgery in treatment of benign breast tumor. Chinese Journal of General Surgery 2011;14:150-1.
- Wang Y, Zhang A, Huang K, et al. Contrastive research on ultrasound guided mammotome minimally invasive surgery and traditional treatment of benign breast lesion. Chinese Journal of Cancer Prevention and Treatment 2010;17:615-8.
- Gao J, Xu S, Liu J, et al. Comparative study of traditional excision and mammotome system in treatment of benign breast lesion(report of 83 cases). Harbin Pharmaceutical 2009;29:16-7.
- Wang J, Chen X. Comparative study of traditional excision and mammotome system minimally invasive excision in treatment of breast fibroadenoma. Journal of Ultrasound in Clinical Medicine 2010;12:700-2.
- Gu B, Zhe D. A clinical study of mammotome minimally invasive system in treatment of nonpalpable breast lesions. China Medical Herald 2008;5:38-9.
- Wang Q, Liang J. A clinical study and evaluation the results of ultrasound guided mammotome minimally invasive biopsy system and regional resection for the treatment of benign breast tumors. Shenyang: China Medical University, 2006:6-9.
- Costantini R, Sardellone A, Marino C, et al. Vacuum-assisted core biopsy (Mammotome) for the diagnosis of non-palpable breast lesions: four-year experience in an Italian center. Tumori 2005;91:351-4. [PubMed]
- Fine RE, Boyd BA, Whitworth PW, et al. Percutaneous removal of benign breast masses using a vacuum-assisted hand-held device with ultrasound guidance. Am J Surg 2002;184:332-6. [PubMed]
- Sperber F, Blank A, Metser U, et al. Diagnosis and treatment of breast fibroadnomas by ultrasound-guided vacuum-assisted biopsy. Arch Surg 2003;138:796-800. [PubMed]
- Plantade R, Hammou JC, Gerard F, et al. Ultrasound-guided vacuum-assisted biopsy: review of 382 cases. J Radiol 2005;86:1003-15. [PubMed]
- Zhang N, Zhang B. Diagnosis and differential diagnosis of benign breast lesion. Chinese Journal of Practical Surgery 2009;29:202-4.