Combined associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) followed by left trisectionectomy and Whipple operation for PNET
Introduction
Pancreatic neuroendocrine tumor (PNET) is slow-growing (1), and account only for 2% of all pancreatic primary tumors. Surgical resection is still the only curative treatment for PNET patients. Unfortunately, most of PNETs was found with unresectable multiple liver metastases and extrahepatic metastasis as their characteristics of non-functional and asymptomatic. With advances in liver surgery in these years, especially combined associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), provide a new curative surgical treatment for PNET with liver metastases patient. Here we reported a case of a 36-year-old man who was diagnosed PNET with liver metastases and underwent ALPPS (followed by left trisectionectomy) and Whipple operation within one-stage.
Case presentation
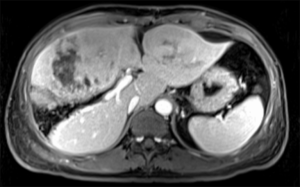
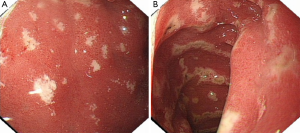
We report on a 37-year-old male patient (176 cm, 68 kg, good past medical history) who presented uncharacteristic symptoms (i.e., epigastric pain, diarrhea, weight lost) 2 months prior to admittance, which led to the initiation of diagnostic procedures by a gastroenterologist and included an esophagogastroscopy, abdominal computed tomography (CT) (Figure 1) and PET-CT. The esophagogastroscopy revealed multiple ulcers of the gastric antrum and duodenum (Figure 2). Based on the CT imaging studies bilobular hepatocellular carcinoma (HCC) was diagnosed, although the HBVs Ag is negative and the serum tumor marker alpha fetoprotein is normal. Other suspicious for metastatic disease (i.e., pancreas, prostate, colon), were excluded by CT and PET-CT. In addition, the serum tumor marker CEA, CA19-9, and prostate-specific antigen were within normal reference range. Besides the patient was feeling fine with no clinical symptoms of diabetes, hypoglycemia, hypopituitarism, hypoparathyroidism.
Clinical course—hepatic surgery (ALPPS stage 1)
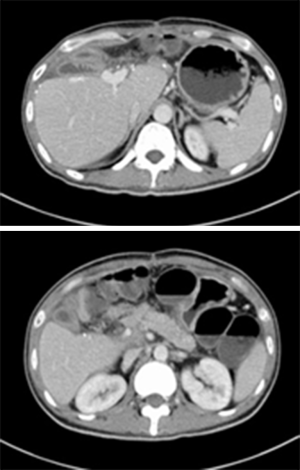
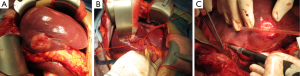
After routine exploration for abdominal surgery, we fully mobilized the liver and performed intraoperative ultrasound (IOUS) in order to evaluate resectability of the hepatic lesions. The hepatic lesions identified by IOUS were resected by multiple sub-segmental resections (n=6, SI/II/III/IVa/IVb/V/VIII). As calculating the future liver remnant (FLR)/estimated standard liver volume (ESLV) ratio just 24%, significant lower than our standard of 30% (no cirrosis). ALPPS stage 1 was performed, during the operation we ligated the left portal vein and partition the SVI/VII from SV/VIII by CUSA (Cavitron Ultrasonic Surgical Aspirator, Valleylab, Boulder, CO, USA). One additional lesion (2 cm) in SIVb and some enlarged lymph nodes in ligamentum hepatoduodenal was resected for pathologic diagnosis (Figure 3A-C).
Clinical course—diagnosis correcting
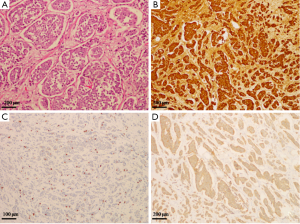
The ALPPS stage 1 hepatic surgery postoperative course was uneventful, with no evidence of bleeding, bile leakage and liver failure. On day 10, the histopathological examinations of the resected liver specimen and the lymph nodes in ligamentum hepatoduodenal revealed metastases of a NET. Microscopically, both the specimen showed small-sized ovoid cells arranged in nests separated by broad fibrous bands and glandular duct formation. They displayed poorly differentiated with large nuclei. Immunohistochemically, the tumors were positive for chromogranin A, synaptophysin, Ki-67 (with index about 5%), but negative for CD56 (Figure 4).
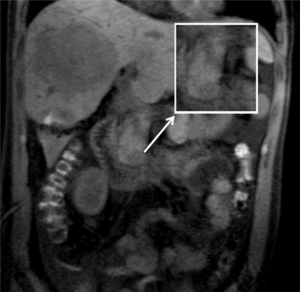
As the primary liver NET is rare, and most primary NET come from pancreas. We performed an emergency MRI examination which identified a small size lesion located at the pancreatic head (Figure 5). In general, corresponded to the resected metastatic liver NET, the lymph nodes in ligamentum hepatoduodenal and the MRI image, we corrected the diagnosis with PNET with liver metastases.
Clinical course—stage 2 surgery (ALPPS stage 2 combine Whipple procedure)
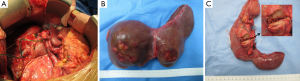
As the patient is young, and recover very smoothly from the stage 1 operation, the SVI/VII volumetry (FLR/ESLV) from 24% increase to 38% (Figure 6), we performed ALPPS stage 2 (left trisectionectomy) combine Whipple procedure at the same time (Figure 7A). During operation pancreatic head tumor identified by IOUS and frozen biopsy section. The histopathological examinations of the resected liver specimen (Figure 7B) revealed metastases of a NET, that, in general, corresponded to the resected primary lesion in the pancreatic head (Figure 7C). Both the primary pancreatic tumor and the liver metastases showed the same typical neuroendocrine tumor.
Follow up
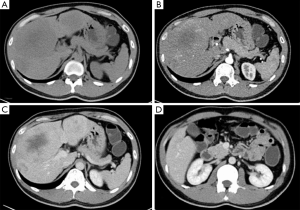
Four years passed, the patient did not accept chemotherapy as patient’s personal reasons. There was no evidence for NET recurrence within the liver or pancreas by CT or MRI (once per 6 months) (Figure 8), the patient general condition is good, diarrhea symptom also improved obviously.
Discussion
At present, operation performing to resect the primary pancreatic tumor and all the metastatic hepatic tumor is the only curative treatment for PNET with liver metastases (2), gives 5-year survivals of 76% (nonresectable 5-year survivals are 30–40%) (3). Typically, CT is the first line imaging choice for most patients with suspected PNET with liver metastases. Most liver metastases of PNET is characterized by rich blood supply from the hepatic artery, so it presents markedly enhancement in the arterial phase. As be lack of the portal vein blood supply, it also present phenomenon of “wash out”, which make it difficult to distinguish PNET with liver metastases from other diseases such as primary liver cancer. In this case, we also misdiagnose it primary HCC at first. On the other hand, in about 11–14% of liver metastases, the primary lesion is hard to even cannot be identified as their small size or location (i.e., pancreas, small bowel, prostate) (4). Just like this case we report, NETs are often present in an advanced stage with an unknown primary or initial diagnosed with metastasis (5,6). For the PNET liver metastasis patients, besides the resection of liver metastatic lesions, it is more important to find the primary site of pancreas, because it has great impact on the patient’s outcome by resection of the primary pancreatic tumor (4). We should be aware of, primary liver-NET, is an extremely rare occurrence, and until 2015, there were only less than 150 reported cases in the English literature (7). So, once we detected PNET in liver, ought to try to identify the primary for an optimal therapy. Over the last few years the detection rate of primary NET in the pancreas by MRI diffusion weighted imaging (DWI) has been improved (8). Recently, somatostatin receptor PET/CT scan technique was one of topics of most concern, as studies reported it can detects about 59% unknown NET primaries. However, which means, even by using such a sensitive technique, more than 1/3 of patients’ primaries could not be found (9).
Liver transplantation (LT) for unresectable metastases has essentially been abandoned as its high rate of tumor recurrence (10). Without exception, several reports showed poor results on LT apply to PNET patients with liver metastases between 1960 and the 1980s. In the present critical situation of donor shortage, LT apply to PNET patients with liver metastases should be strictly controlled. It has been wildly accepted that only highly selected PNET patients with liver metastases may be candidates for LT (11-13). The only prospective study recommended strict selection criteria (i.e., low grade, removal of primary tumor, liver involvement <50%, age <55 years, and stable disease for ≥6 months before LT) for LT with curative treatment (13). These strict selection criteria have strongly limited the surgical treatment for PNET patients with liver metastases until ALPPS was reported as a novel surgical technique liver resection in traditionally nonresectable primary intrahepatic tumors or metastatic liver tumors since 2012 (14-16).
For those patients with hepatic mass and small-size FLR, ALPPS provide a novel resolution. The procedure is consisted of two staged hepatectomy by which rapid significant hypertrophy of the remnant is induced by transecting the parenchyma while there is not enough time for the development of collateral formation (16-21). The technique is relied on portal vein ligation and in situ splitting through parenchyma so that the two parts are just connected through hilar structures in the first step. Partitioning will be completed after an interval of 7 days if FLR hypertrophy is satisfactory (15,19). Particularly worth mentioning is the NET patients’ liver is normal, which always contribute a rapid increase of FLR hypertrophy, are good cases for this procedure.
Only very few literatures compare the outcome of one-stage or two-stage hepatectomy for metastatic liver tumor in patients undergoing Whipple operation, a dual center analysis showed the advantage of less abscess incidence (7%) in one-stage operation group (22). To our knowledge, by retrieving literatures, we find no literature on the ALPPS combine Whipple operation for PNET within one-stage.
In conclusion, combined ALPPS and Whipple operation within one-stage appears to be a valuable option for patients with advanced pancreatic NETs metastatic to the liver, thus facilitating curative liver surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Kimura W, Tezuka K, Hirai I. Surgical management of pancreatic neuroendocrine tumors. Surg Today 2011;41:1332-43. [Crossref] [PubMed]
- Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 2014;15:e8-21. [Crossref] [PubMed]
- Berardi R, Rinaldi S, Torniai M, et al. Gastrointestinal neuroendocrine tumors: Searching the optimal treatment strategy--A literature review. Crit Rev Oncol Hematol 2016;98:264-74. [Crossref] [PubMed]
- Bergsland EK, Nakakura EK. Neuroendocrine tumors of unknown primary: is the primary site really not known? JAMA Surg 2014;149:889-90. [Crossref] [PubMed]
- Morris GJ, Greco FA, Hainsworth JD, et al. Cancer of unknown primary site. Semin Oncol 2010;37:71-9. [Crossref] [PubMed]
- Stoyianni A, Pentheroudakis G, Pavlidis N. Neuroendocrine carcinoma of unknown primary: a systematic review of the literature and a comparative study with other neuroendocrine tumors. Cancer Treat Rev 2011;37:358-65. [Crossref] [PubMed]
- Mousavi SR, Ahadi M. Primary Neuroendocrine Tumor of Liver (Rare Tumor of Liver). Iran J Cancer Prev 2015;8:e3144. [Crossref] [PubMed]
- Brenner R, Metens T, Bali M, et al. Pancreatic neuroendocrine tumor: added value of fusion of T2-weighted imaging and high b-value diffusion-weighted imaging for tumor detection. Eur J Radiol 2012;81:e746-9. [Crossref] [PubMed]
- Prasad V, Ambrosini V, Hommann M, et al. Detection of unknown primary neuroendocrine tumours (CUP-NET) using (68)Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging 2010;37:67-77. [Crossref] [PubMed]
- Chapman WC. Liver transplantation for unresectable metastases to the liver: a new era in transplantation or a time for caution? Ann Surg 2013;257:816-7. [Crossref] [PubMed]
- Gottwald T, Koveker G, Busing M, et al. Diagnosis and management of metastatic gastrinoma by multimodality treatment including liver transplantation: report of a case. Surg Today 1998;28:551-8. [Crossref] [PubMed]
- Le Treut YP, Gregoire E, Klempnauer J, et al. Liver transplantation for neuroendocrine tumors in Europe-results and trends in patient selection: a 213-case European liver transplant registry study. Ann Surg 2013;257:807-15. [Crossref] [PubMed]
- Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol 2007;47:460-6. [Crossref] [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. [Crossref] [PubMed]
- Donati M, Stavrou GA, Oldhafer KJ. Current position of ALPPS in the surgical landscape of CRLM treatment proposals. World J Gastroenterol 2013;19:6548-54. [Crossref] [PubMed]
- Aloia TA, Vauthey JN. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): what is gained and what is lost? Ann Surg 2012;256:e9; author reply e16-9.
- Yaprak O, Guler N, Altaca G, et al. Ratio of remnant to total liver volume or remnant to body weight: which one is more predictive on donor outcomes? HPB (Oxford) 2012;14:476-82. [Crossref] [PubMed]
- Torres OJ, Fernandes Ede S, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): the Brazilian experience. Arq Bras Cir Dig 2013;26:40-3. [Crossref] [PubMed]
- de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the "ALPPS" approach. Ann Surg 2012;255:415-7. [Crossref] [PubMed]
- Andriani OC. Long-term results with associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Ann Surg 2012;256:e5; author reply e16-9.
- Vennarecci G, Laurenzi A, Santoro R, et al. The ALPPS procedure: a surgical option for hepatocellular carcinoma with major vascular invasion. World J Surg 2014;38:1498-503. [Crossref] [PubMed]
- De Jong MC, Farnell MB, et al. Liver-directed therapy for hepatic metastases in patients undergoing pancreaticoduodenectomy: a dual-center analysis. Ann Surg 2010;252:142-8. [Crossref] [PubMed]