Short and long-term impact of parathyroid autotransplantation on parathyroid function after total thyroidectomy
Introduction
Postsurgical hypocalcaemia due to hypoparathyroidism is the most frequent and occasionally the most serious complication of total thyroidectomy (1). Inadvertent compromise of parathyroid blood supply or unintentional excision of parathyroid glands is the usual etiology of hypoparathyroidism (2). Parathyroid autotransplantation provides a solution to intraoperative parathyroid compromise, despite common utilization, the impact of autotransplantation on parathyroid function remains incompletely understood.
The symptoms of hypocalcaemia due to post-operative hypoparathyroidism usually present in the first 24–72 hours after surgery (3). Features of hypocalcaemia include circumoral or peripheral paraesthesia, tetany, carpopedal spasm, laryngospasm and ECG changes progressing from long QT interval to VT arrest (4). In addition to patient morbidity and mortality, post-thyroidectomy hypocalcaemia leads to greater healthcare costs due to the need for extra biochemical testing, electrolyte supplementation and increased length of hospital stays (5).
In the longer term, undiagnosed hypoparathyroidism can lead to multiple systemic sequelae such as chronic renal impairment, reduced bone remodeling, increased psychiatric complaints and basal ganglia calcification (6). This is associated with significant morbidity and reduced quality of life. A recent population study by Hadker et al. demonstrated that 75% of sufferers of chronic hypoparathyroidism experienced symptoms despite treatment, 79% had required emergency department or hospital admissions and 85% reported inability to perform household tasks (7).
The recognition of the disease burden associated with permanent hypoparathyroidism has led to the emergence of parathyroid autotransplantation as a means to reduce the prevalence of this complication in at risk patients. Parathyroid autotransplantation was first described and performed in humans during a thyroidectomy by Lahey (8) in 1926. The procedure was largely forgotten for 50 years until Wells et al. (9) reported the first patient series that confirmed functional autografts clinically, physiologically and histologically.
Burden of disease and defining parathyroid failure
Part of the difficulty in defining the role of parathyroid autotransplantation relates to the variability in incidence and presentation of hypoparathyroid disease. Reported incidences of transient and permanent hypocalcaemia are highly variable, ranging from 10–61% (10,11) and 1–32% (12-15), respectively. In a more recent meta-analysis the estimated incidence of transient and permanent hypocalcaemia was 19–38% and 0–3%, respectively (16). National registries and large multicenter studies however, consistently demonstrate higher rates of permanent hypocalcaemia ranging from 6.4–9% (1,17) with the Fifth National Audit Report from the British Association of Endocrine and Thyroid Surgeons demonstrating a rate of 6.5% (18), having dropped from 12.1% in 2012 (19). The variable rates of hypoparathyroidism and concomitant hypocalcaemia in the literature partly relates to inconsistent definitions of hypoparathyroidism, hypocalcaemia and the division between transient and permanent conditions (20). A recent paper by Mehanna et al. described the reported rate of postoperative hypocalcaemia for the same cohort of patients varied 46-fold depending on the definitions used (21).
Lorente-Poch et al. (22) attempted to address the diagnostic confusion by defining three distinct syndromes of parathyroid failure post total thyroidectomy. This consisted of (I) “postoperative hypocalcemia”: a serum calcium of <2 mmol/L or <8 mg/dL within 24 hours after surgery requiring calcium/vitamin D replacement therapy at the time of hospital discharge; (II) “protracted hypoparathyroidism”: a subnormal iPTH concentration <13 pg/mL and/or need for calcium/vitamin D replacement at 4–6 weeks; (III) “permanent hypoparathyroidism”: subnormal iPTH concentration <13 pg/mL and/or need for calcium/vitamin D replacement one year after total thyroidectomy.
The implementation of standardized definitions for hypocalcaemia would greatly improve the quality of literature and improve our understanding of the role of parathyroid autotransplantation in preventing hypoparathyroidism after thyroid surgery.
Risk factors for hypoparathyroidism and prevention of hypoparathyroidism
The earliest thyroid operations were performed in the mid-1800s. However, at this early juncture, procedures had such a poor outcome that Samuel Gross wrote in 1866 ‘if a surgeon should be so adventurous or foolhardy as to undertake thyroidectomy, every step he takes will be environed with difficulties’ (23). Nevertheless, by the end of the 1800s Professor Theodore Kocher had reported 900 thyroid procedures with a mortality of merely 1% and minimal morbidity. However, Kocher’s case mix included only 18 total thyroidectomies, as he had found that his post-thyroidectomy patients did poorly, developing a condition he termed ‘cachexia strumipriva’, in actuality, hypoparathyroidism. This lead Professor Kocher to abandon total thyroidectomy in favor of subtotal procedures (24).
Since then and particularly the last 30 years, improvements in surgical technique such as the advent of capsular dissection (23), careful handling of the parathyroid blood supply, loupe magnification (25) and truncal ligation of the inferior thyroid artery have rendered total thyroidectomy safer (Figure 1), allowing modern surgeons to harness the benefits of total thyroidectomy over subtotal approaches such as reduced recurrence for benign disease and increased survival for malignancy. Consequently, total thyroidectomy has become a preferred operation (23,26-28) for many benign and malignant thyroid pathologies. In 2015, Antakia et al. (29) underwent an extensive systematic review and meta-analysis to evaluate the effectiveness of surgical measures on post-operative hypocalcaemia. This found that less extensive surgery, such as subtotal thyroidectomy, is associated with less risk of transient hypocalcaemia but felt that this did not influence the argument regarding extent of thyroidectomy as there was no impact regarding permanent hypocalcaemia.
Recently Edafe et al. (16) undertook a systematic review of predictors for post thyroidectomy hypocalcaemia and found female gender, preoperative calcium, perioperative PTH levels, surgery for recurrent goiter or Grave’s disease, reoperation for bleeding, inadvertent parathyroid gland excision, and parathyroid autotransplantation to be predictors for transient hypocalcaemia. Predictors for permanent hypocalcaemia included a calcium level lower than 1.88 mmol/L 7.54 mg/dL at 24 h after surgery, identification of fewer than two parathyroid glands at surgery, reoperation for bleeding, Graves’ disease and heavier thyroid specimens. They noted that no study included in the review found parathyroid autotransplantation to be a predictor for permanent hypocalcaemia.
Any factor that compromises parathyroid gland function is a potential risk factor for post-operative hypoparathyroidism (2,4,11,30). Hence, the extent of surgery, inadvertent excision of parathyroid glands and identification of viable glands are repeatedly demonstrated to be factors in post-operative hypoparathyroidism (16,17,31,32). The incidence of incidental parathyroidectomy during thyroidectomy can be as high as 17.7% (32). Selective parathyroid autotransplantation with functional grafted parathyroid tissue theoretically should negate other factors causing dysfunction. However, it has also been demonstrated that the number of parathyroid glands remaining in situ is a critical factor in prevention of permanent hypoparathyroidism (33). Therefore, the success of parathyroid autotransplantation relies on the recognition of compromised parathyroid glands while maintaining the function of parathyroid glands left in place (Table 1).

Full table
Identifying devascularized glands
Parathyroid glands are usually identified by experienced surgeons through subjective visual assessment and detailed knowledge of anatomy (34). With appropriate training, Endocrine surgeons have been shown to develop satisfactory skills for the visual identification of viable parathyroid glands (35). Sung et al. (36) found that if at least one parathyroid gland was deemed to have a normal appearance at the time of closure then the development of hypocalcaemia requiring treatment is uncommon. However, it has also been demonstrated that even with meticulous dissection, anatomically intact glands may not be physiologically viable due to thrombosis of the delicate parathyroid vascular pedicle, or parathyroid capsule oedema (37). This is reinforced by Lang et al. (38) who found that parathyroid gland dusky discoloration was associated with transient hypoparathyroidism but also found that apparently normal glands with seemingly intact vascularity does not always imply that the gland is functional.
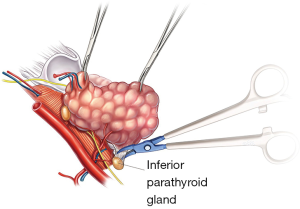
The most straightforward indication for parathyroid autotransplantation is when parathyroid glands are inadvertently removed with the thyroid specimen or when glands are obviously devascularized. Novel fluorescence techniques are currently under evaluation to improve parathyroid identification during thyroidectomy. The parathyroid glands exhibit significantly higher fluorescence intensity than surrounding tissues and this has been suggested as a tool for identification of parathyroid tissue (39,40). However, fluorescence only has the benefit of improving gland localization, it does not indicate that the gland remains viable after dissection. For this reason, some studies advocate for a ‘knife test’ of questionable glands checking for capillary bleeding (41), this has the added benefit of avoiding venous congestion in the gland, yet there is some concern that this may compromise viable glands. Routine frozen section of potential parathyroid tissue is used in some centers to confirm parathyroid tissue prior to transplantation (42). However Lo and Lam (43) determined that this was unnecessary for experienced surgeons as they felt unquestionable macroscopic appearance negated the need for frozen sections which risked reducing the amount of parathyroid tissue available for transplantation. There has also been an initial report regarding use of doppler ultrasound however clinically this is not easily feasible intraoperatively (44).
Perhaps the most useful assessment of parathyroid function intraoperatively is use of parathyroid hormone immunoassay. Barczyński et al. (45) demonstrated that taking a PTH level 10–20 minutes after total thyroidectomy prior to closure and utilizing a level <10 ng/L as an indicator for parathyroid autotransplantation resulted in a lower incidence of transient hypocalcaemia and reduced the risk of permanent hypothyroidism. This was also cost effective, being calculated to cost $35 USD and time effective with results available within 8–10 minutes. Utilizing parathyroid level biochemistry and visual assessment of incidentally removed or discolored compromised glands as a guide to parathyroid auto transplant may reduce the incidence of transient hypoparathyroidism while mitigating the risk of permanent hypothyroidism.
Technique of autotransplantation
There are various techniques for autotransplantation, these include thin section slicing, mincing and injection of a suspension of parathyroid tissue (9,42,46,47). The most widely utilized technique for parathyroid autotransplantation is described by Wells et al. (9) In this approach, parathyroid tissue is placed in saline at 4 °C or in tissue culture medium as soon as possible after excision. After cooling for 30 min, the glands are sufficiently firm to be sliced into 1 mm slices or 1 mm cubes. Generally, 10–20 pieces are inserted into individual muscle pockets of muscle. The incision is closed with a non-absorbable suture or clip to assist subsequent identification.
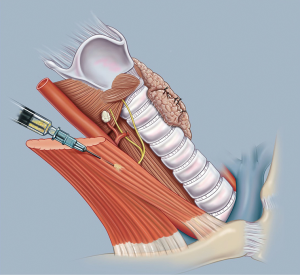
Another common technique involves injection of a suspension of parathyroid tissue in buffered saline into the sternocleidomastoid muscle as demonstrated in Figure 2. This is our preferred technique and one which we have found to be reliable and easy to perform. We use 1–2 mL of sterile physiological salt solution such as BSS and carefully mince the de-vascularized parathyroid gland using fine scissors. The small fragments of tissue are drawn into a syringe and the suspension is injected into the right sternomastoid muscle at its midpoint. This produces comparable return of parathyroid outcome when compared to thin sectioning or mincing (46). This technique should be avoided when there may be a need for subsequent excision of part of the graft, as injected parathyroid tissue is diffuse and difficult to localize for re-excision. When surgery is undertaken for Multiple Endocrine Neoplasia Types 1 and 2, or renal failure patients with secondary and tertiary hyperparathyroidism this approach should be avoided. On the other hand, the relative ease of this technique makes it the procedure of choice for parathyroid autotransplantation during thyroidectomy in general cases.
Site for auto transplant
There are many different potential sites for re-implantation of parathyroid tissue including the sternocleidomastoid, brachio-radialis, pectoralis major muscles (48) and, more recently, into the subcutaneous tissue of the forearm (49). Most common in total thyroidectomy is the sternocleidomastoid muscle which usually already lies exposed in the operative field (44,50,51). As the parathyroid glands are expected to be functionally normal in the vast majority of cases of thyroid surgery this is a safe and easily accessible area. However, it is not possible to biochemically prove graft hyper-function when implanted into the neck. If there was a future need for removal of the graft, then re-operative surgery requires repeat cervical exploration.
Hence, if parathyroid autotransplantation is indicated for abnormal or hyperplastic parathyroid tissue, then the preferred graft location is usually in the brachio-radialis muscle of the non-dominant forearm (52). Sampling of venous return in the forearm containing the graft allows checking of graft function or dysfunction. A gradient of 1.5 or greater in PTH measurement between grafted and non-grafted arms has been generally accepted as proof of graft hyper-function (53). Additionally, if removal of the graft is indicated, the potential difficulties and risks associated with redo cervical surgery are alleviated. Although rare, auto transplanted parathyroid tissue has been demonstrated to result in primary hyperparathyroidism even after grafting of normal parathyroid tissue in total thyroidectomy (54). Utilizing, the brachio-radialis muscle has been successfully used by Lo and Tam to demonstrate parathyroid graft function after autotransplantation for total thyroidectomy (53).
More recently, Cavallaro et al. (49) have demonstrated parathyroid autotransplantation into forearm subcutaneous tissue to be a safe and effective procedure when used for normal functioning parathyroid glands inadvertently removed during total thyroidectomy. This had previously been documented to be effective in parathyroid hyperplasia (55). In a small study of 25 cases, they demonstrated recovery of reimplanted glands in 96% of cases at three months. This technique is faster and less invasive than intramuscular techniques, requiring only 2 mm skin excisions.
Forearm implantation provides an effective alternative to the traditional technique of reimplantation into the sternocleidomastoid muscle, and has the added benefit of easier monitoring of graft function and subsequent removal if graft dysfunction ensues. However, it is more time consuming due to the need for secondary incisions at a distant site. For this reason, the sternomastoid muscle with its ease and proximity, is the preferred site for auto-transplantation of parathyroid tissue during thyroidectomy.
Results of parathyroid autotransplantation
Parathyroid autotransplantation has been demonstrated to be effective, clinically and biochemically, with a functional graft survival rate of 93% (9). However, the clinical utility of parathyroid autotransplantation following thyroid surgery is more difficult to evaluate, because it is a rare event for all four parathyroid glands to be removed or devascularized during thyroidectomy. Indeed, Song et al. found that even one remaining functional gland in situ provides a safe guard against permanent hypoparathyroidism; although the incidence of transient hypocalcaemia correlates with the number of parathyroid glands damaged or removed.
The question which arises is whether parathyroid autotransplantation should be performed when only one or two parathyroid glands have been removed accidentally or suspected to be devascularized. In other words, is there any additional benefit of parathyroid gland re-implantation in the likely presence of at least one functional in situ parathyroid gland? Delbridge (23) suggested that “the viability of in situ vascularized parathyroid remains unpredictable with late ischemia always a possibility. The routine autotransplantation of at least one parathyroid gland during every total thyroidectomy, while unnecessary in most cases, provides insurance in cases where late ischemia of the remaining glands actually occurs.” (23) Zedenius et al. (41) demonstrated this, showing that routine autotransplantation of one parathyroid gland during total thyroidectomy eliminated permanent hypoparathyroidism in a study of 100 consecutive total thyroidectomies.
Conversely, Lo and Lam (12) examined a policy of routine parathyroid autotransplantation compared to selective autotransplantation and found no significant difference in the rates of permanent hypoparathyroidism, 0% compared to 1.8% respectively but a significantly higher rate of transient hypocalcaemia, 23% versus 13%. This is reinforced by Barczynski et al. (45) who utilized PTH assay intraoperatively to guide parathyroid autotransplantation and found that selective autotransplantation yielded a lower incidence of transient hypocalcaemia when compared to routine autotransplantation, 11.2% versus 22.4% respectively. Both approaches yielded a 100% success rate in preventing permanent hypoparathyroidism. Thus, routine autotransplantation although still highly effective in preventing permanent hypoparathyroidism likely increases transient parathyroid dysfunction which extends hospital stays, causing discomfort and potentially life-threatening complications and could be avoided if a normal well vascularized gland had remained in situ.
Although some studies appear to demonstrate lower rates of permanent hypoparathyroidism after parathyroid autotransplantation compared to controls, most of these were underpowered and failed to demonstrate statistically significant differences. On the other hand, some scholars have argued that parathyroid autotransplantation places potentially viable parathyroid glands at risk, and have cautioned against routine autotransplantation. Lorente-Poch et al. (33) measured the parathyroid glands remaining in situ score (PGRIS), and found this inversely related to the rate of permanent hypoparathyroidism. However, this score included both auto transplanted and removed glands, thereby not directly testing function of the grafted parathyroid gland. A further study by the same authors found that the rates of permanent hypoparathyroidism were similar whether the glands are incidentally resected or auto transplanted. In a larger population study (56), parathyroid autotransplantation significantly increased the risk of transient hypoparathyroidism, OR 5.19, P value <0.0001, whilst it showed a non-statistically significant trend toward minimizing the risk of permanent hyperparathyroidism.
Parathyroid autotransplantation has repeatedly been found to be a risk factor for transient hypocalcaemia (11,12,16,56-58). Edafe et al. (16) conducted a systematic review and meta-analyses of predictors of post-operative hypocalcaemia and found that patients undergoing parathyroid autotransplantation had double the risk of transient hypocalcaemia. However, these studies instituted selective autotransplantation only in cases when the blood supply to a gland was deemed to be questionable, which in turn were compared against controls in which auto-transplantation had not been indicated. Therefore, on the basis of this evidence, it cannot be answered if parathyroid gland autotransplantation is an independent risk factor. Additionally, Testini et al. (51) found that parathyroid autotransplantation reduced the incidence of transient hypocalcaemia when they compared age, sex and surgical indication matched controls with one devascularized gland, to patients undergoing parathyroid autotransplantation due to having one devascularized gland. Therefore, despite the strong correlation between autotransplantation and transient hypocalcaemia, these results may be partially confounded by the indication for autotransplantation being parathyroid devascularization.
Two studies have demonstrated a statistically significant decrease in permanent hypoparathyroidism after parathyroid autotransplantation. Ahmed et al. (59) demonstrated a 3.09% incidence of permanent hypocalcaemia in patients with no parathyroid autotransplantation and a 0.34% incidence in patients following autotransplantation with a P value of 0.0417. The patients in this study were initially randomly assorted but favorable results in the autotransplantation group led to a policy of routine autotransplantation, this demonstrates a bias towards the use of parathyroid grafting. Wei et al. (60) examined the role of selective autotransplantation of inferior thyroid glands in patients undergoing total thyroidectomy and central neck dissection. They found that permanent hypoparathyroidism was lower after parathyroid autotransplantation compared with preservation in situ, 0.9% and 3.8% respectively with a P value of 0.003. The authors also note that autotransplantation may allow a more comprehensive central neck dissection. This may illustrate that parathyroid autotransplantation is more effective in cases where central neck dissection is required as these have been demonstrated to have an increased risk of post-operative hypocalcaemia (29). Additionally, a large retrospective cohort study of 1,196 patients demonstrated that using a selective policy of autotransplantation maintains a rate of permanent hypoparathyroidism at less than 1% (11). However, no significant difference was found between patients undergoing parathyroid transplantation or controls.
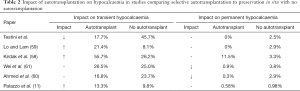
Overall parathyroid autotransplantation is associated with transient hypoparathyroidism but a reduction in rates of permanent hypoparathyroidism. Although the number of patients benefitted will be low, as only one functioning gland in situ may be enough for prevention, the morbidity related to permanent hypocalcaemia is so significant that this small potential benefit is more advantageous than leaving devascularized or jeopardized glands in place where their ongoing function is unpredictable. Despite a lack of clear empirical evidence, parathyroid autotransplantation is an effective procedure that can safeguard against permanent hypoparathyroidism (Table 2).
Full table
Cryopreservation
Cryopreservation is another method, again pioneered by Wells (61), to avoid permanent hypoparathyroidism following parathyroid or thyroid surgery (9,62). Cryopreservation involves the freezing and storage of parathyroid tissue in case permanent hypoparathyroidism occurs (63). It has been advocated in patients undergoing surgery for persistent or recurrent hyperparathyroidism and patients requiring subtotal or total parathyroidectomy but also in thyroid and central neck dissection due to the risk of failure of immediately transplanted autografts (64). Although its most obvious indication remains primary multi-glandular hyperparathyroidism when it is unclear if post-operative patients will go on to develop further hyperparathyroidism but remain at considerable risk of developing permanent hypoparathyroidism postoperatively (65).
Transplantation of cryopreserved tissue is less effective than immediate autotransplantation with a highly variable reported success rate from 10–100% (64-66). Agarwal et al. (66) cryopreserved 630 specimens following parathyroid surgery, and reported a 100% success rate as measured by an increase in circulating parathyroid hormone from 9 delayed parathyroid transplantation procedures utilizing stored parathyroid tissue. However, 2 of the patients remained on high dose calcium at the conclusion of their study. They did not offer parathyroid gland cryopreservation for routine thyroidectomy patients. On the other hand, a large multicenter trial in France cryopreserved 1,376 parathyroid tissue samples. Of these, only 22 were auto grafted into 20 patients, of which only 10%, were fully functional and another 10% retained partial function with patients requiring ongoing calcium and vitamin D supplementation.
Cryopreservation is a useful and potentially beneficial procedure in only a very small number of selected cases of permanent hypocalcaemia. The benefit of retaining parathyroid tissue for cryopreservation following the majority of cases of thyroidectomy remains doubtful, given that rates of permanent hypoparathyroidism are below 1% when selective immediate parathyroid autotransplantation is utilized (11).
New techniques and the future of treatment for permanent hypoparathyroidism
Most studies continue to focus on prevention of parathyroid dysfunction by careful preservation in situ and utilizing selective parathyroid autotransplantation when necessary. Yet the disease burden of post-operative hypocalcaemia remains high with many studies reporting a low level of permanent hypocalcaemia despite utilizing autotransplantation. Considering the relative success of parathyroid autotransplantation and the innate characteristic of parathyroid tissue to be transplanted (67) it is worth considering allotransplantation in the treatment of chronic hypoparathyroidism.
Unfortunately, to date, allotransplantation has had variable results usually due to rejection by alloimmunization or inflammatory responses causing fibrosis compromising graft survival (68,69). The risks involved in immunosuppression both from medication side effects and risk of infection are generally not considered to outweigh the benefit in terms of long term treatment for hypoparathyroidism. Yet Agha et al. argue in a recent case study that this needs to be considered on an individual basis after demonstrating successful allotransplantation in a 30-year-old with post-operative permanent hypocalcaemia who had failed medical therapy. Alternatively, Nawrot et al. (70) demonstrated that it is possible to culture non immunogenic parathyroid cell populations. They found that of 85 allotransplants, 65 cellular allografts retained endocrine function for two months with a mean overall graft survival of 6.3 months. Graft function was considered present when overall PTH level increased compared to pre-operatively, the was an evident PTH gradient between ipsilateral and contralateral arms and patients no longer required vitamin D and only minimal calcium supplementation. Case reports have also demonstrated increased survival through the use of microencapsulated parathyroid tissue. Cabané et al. (71) demonstrated a 20-month graft survival in a patient previously needing continuous intravenous calcium for survival. Therefore, parathyroid allotransplantation is a potential option for long term hypoparathyroidism however it doesn’t negate the importance of in situ preservation and parathyroid autotransplantation in total thyroidectomy.
Alternatively, new stem cell research has yielded promising results regarding the generation of parathyroid-like cells. Ignotski et al. and Bingham et al. have demonstrated a method of generating cells producing parathyroid hormone RNA and parathyroid hormone from two different human embryonic stem cell lines (72,73) utilizing activin A and soluble sonic hedgehog. They note that parathyroid cells are optimal for cellular replacement as each cell contains that complete function of the organ, no architectural structure is needed for parathyroid cells to resume function and autotransplantation has been demonstrated to reconstitute normal parathyroid function. In vitro studies in rats’ post parathyroidectomy have demonstrated survival benefit after implantation of differentiated human tonsil-derived mesenchymal stem cells (TMSC) (74,75). However, these studies are short term and demonstrate limited survival benefit with a 40% increase in mortality at 28 days and 50% at three months. Therefore, more research is required before stem cells differentiated to produce parathyroid hormone are useful in the treatment of post thyroidectomy hypoparathyroidism.
Another potential treatment option is recombinant human PTH which has been accepted for use by the FDA in America in 2015 and in the EU since earlier this year. In a phase III trial, subcutaneous rhPTH 1–84 was effective in maintaining total serum calcium levels while reducing or eliminating the need for oral calcium and active vitamin D (76). However, studies in rats have demonstrated a risk of osteosarcoma (77). As yet, no skeletal malignancies have been described by clinical trials in humans with cumulative numbers of 16,000 subjects treated with up to three years of continuous therapy (78). Currently, recombinant PTH is a promising option in the treatment of permanent hypoparathyroidism however long term studies of ongoing follow up of patients using these medications are needed to further determine their efficacy and safety.
Conclusions
Minimizing the costs and morbidity associated with hypoparathyroidism following thyroid surgery should begin with the implementation of standardized definitions of hypocalcaemia so that individual surgical units can quantify their own rates. A strong emphasis on surgical technique to avoid vascular injury to the parathyroid is critical to good long term outcomes as well as an awareness of the high risk cases which include multi-glandular parathyroid disease and lymph node dissection in which increase there is a greater risk of disrupting parathyroid function. Parathyroid function, and its permanence, correlates with the number of functioning parathyroid glands which remain in situ. The functional viability of parathyroid glands may best be assessed by visual inspection and the use of intraoperative PTH immunoassays. An approach of selective auto-transplantation of de-vascularized glands provides an effective means of restoring parathyroid function and is protective against permanent hypoparathyroidism.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg 2008;393:667-73. [Crossref] [PubMed]
- Tredici P, Grosso E, Gibelli B, et al. Identifcation of patients at high risk for hypocalcemia after total thyroidectomy. Acta Otorhinolaryngol Ital 2011;31:144-8. [PubMed]
- Mowschenson PM, Hodin RA. Outpatient thyroid and parathyroid surgery: a prospective study of feasibility, safety, and costs. Surgery 1995;118:1051-3. [Crossref] [PubMed]
- Shoback DM, Bilezikian JP, Costa AG, et al. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab 2016;101:2300-12. [Crossref] [PubMed]
- Bhattacharyya N, Fried MP. Assessment of the morbidity and complications of total thyroidectomy. Arch Otolaryngol Head Neck Surg 2002;128:389-92. [Crossref] [PubMed]
- Mitchell D, Regan S, Cooley M, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab 2012;97:4507-14. [Crossref] [PubMed]
- Hadker N, Egan J, Sanders J, et al. Understanding the burden of illness associated with hypoparathyroidism reported among patients in the PARADOX study. Endocr Pract 2014;20:671-9. [Crossref] [PubMed]
- Lahey FH. The transplantation of parathyroids in partial thyroidectomy. Surg Gynaecol Obstet 1926;42:508-9.
- Wells SA, Gunnells JC, Shelburne JD, et al. Transplantation of the parathyroid glands in man: clinical indications and results. Surgery 1975;78:34-44. [PubMed]
- Glinoer D, Andry G, Chantrain G, et al. Clinical aspects of early and late hypocalcaemia afterthyroid surgery. Eur J Surg Oncol 2000;26:571-7. [Crossref] [PubMed]
- Palazzo FF, Sywak MS, Sidhu SB, et al. Parathyroid autotransplantation during total thyroidectomy--does the number of glands transplanted affect outcome? World J Surg 2005;29:629-31. [Crossref] [PubMed]
- Lo CY, Lam KY. Routine parathyroid autotransplantation during thyroidectomy. Surgery 2001;129:318-23. [Crossref] [PubMed]
- de Roy van Zuidewjn DB, Songun I, Kievit J, et al. Complications of thyroid surgery. Ann Surg Oncol 1995;2:56-60. [Crossref] [PubMed]
- Harness JK, Fung L, Thompson NW, et al. Total thyroidectomy: complications and techniques. World J Surg 1986;10:781-6. [Crossref] [PubMed]
- Hay ID, Grant CS, Taylor WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery 1987;102:1088-95. [PubMed]
- Edafe O, Antakia R, Laskar N, et al. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014;101:307-20. [Crossref] [PubMed]
- Thomusch O, Machens A, Sekulla C, et al. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 2003;133:180-5. [Crossref] [PubMed]
- Chadwick D, Kinsman R, Walton P. The British association of endocrine & thyroid surgeon. Fifth National Audit Report 2017.
- Chadwick D, Kinsman R, Walton P. The British association of endocrine & thyroid surgeon. Fourth National Audit Report 2012.
- Stack BC Jr, Bimston DN, Bodenner DL, et al. American association of clinical endocrinologists and american college of endocrinology disease state clinical review: postoperative hypoparathyroidism--definitions and management. Endocr Pract 2015;21:674-85. [Crossref] [PubMed]
- Mehanna HM, Jain A, Randeva H, et al. Postoperative hypocalcemia--the difference a definition makes. Head Neck 2009.279-83.
- Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, et al. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg 2015;4:82-90. [PubMed]
- Delbridge L. Total thyroidectomy: the evolution of surgical technique. ANZ J Surg 2003;73:761-8. [Crossref] [PubMed]
- Dorairajan N, Pradeep PV. Vignette thyroid surgery: a glimpse into its history. Int Surg 2013;98:70-5. [Crossref] [PubMed]
- Testini M, Nacchiero M, Piccinni G, et al. Total thyroidectomy is improved by loupe magnification. Microsurgery 2004;24:39-42. [Crossref] [PubMed]
- Barakate M, Agarwal G, Reeve TS, et al. Total thyroidectomy is now the preferred option for the management of GraveS' disease. ANZ J Surg 2002;72:321-4. [Crossref] [PubMed]
- Reeve TS, Delbridge L, Cohen A, et al. Total thyroidectomy. The preferred option for multinodular goitre. Ann Surg 1987;206:782-6. [Crossref] [PubMed]
- Khadra M, Delbridge L, Reeve TS, et al. Total thyroidectomy: its role in the management of thyroid disease. Aust N Z J Surg 1992;62:91-5. [Crossref] [PubMed]
- Antakia R, Edafe O, Uttley L, et al. Effectiveness of preventative and other surgical measures on hypocalcemia following bilateral thyroid surgery: a systematic review and meta-analysis. Thyroid 2015;25:95-106. [Crossref] [PubMed]
- Inversini D, Rausei S, Ferrari C, et al. Early intact PTH (iPTH) is an early predictor of postoperative hypocalcemia for a safer and earlier hospital discharge: an analysis on 260 total thyroidectomies. Gland Surg 2016;5:522-8. [Crossref] [PubMed]
- Cho JN, Park WS, Min SY. Predictors and risk factors of hypoparathyroidism after total thyroidectomy. Int J Surg 2016;34:47-52. [Crossref] [PubMed]
- Sakorafas GH, Stafyla V, Bramis C, et al. Incidental parathyroidectomy during thyroid surgery: an underappreciated complication of thyroidectomy. World J Surg 2005;29:1539-43. [Crossref] [PubMed]
- Lorente-Poch L, Sancho JJ, Ruis S, et al. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 2015;102:359-67. [Crossref] [PubMed]
- Lo CY. Parathyroid autotransplantation during throidectomy. ANZ J Surg 2002;72:902-7. [Crossref] [PubMed]
- Reeve TS, Cutrin A, Fingleton L, et al. Can total thyroidectomy be performed as safely by general surgeons in provincial centers as by surgeons in specialized endocrine surgical units? Making the case for surgical training. Arch Surg 1994;129:834-6. [Crossref] [PubMed]
- Sung TY, Lee YM, Yoon JH, et al. Importance of the intraoperative appearance of preserved parathyroid glands after total thyroidectomy. Surg Today 2016;46:356-62. [Crossref] [PubMed]
- Delbridge L. Parathyroid autotransplantation: an essential technique for safe thyroid surgery. ANZ J Surg 2002;72:852-3. [Crossref] [PubMed]
- Lang BH, Chan DT, Chow FC, et al. The association of discolored parathyroid glands and hypoparathyroidism following total thyroidectomy. World J Surg 2016;40:1611-7. [Crossref] [PubMed]
- Falco J, Dip F, Quadri P, et al. Cutting edge in thyroid surgery: autofluorescence of parathyroid glands. J Am Coll Surg 2016;223:374-80. [Crossref] [PubMed]
- Shinden Y, Nakajo A, Arima H, et al. Intraoperative identification of the parathyroid gland with a fluorescence detection system. World J Surg 2017;41:1506-12. [Crossref] [PubMed]
- Zedenius J, Wadstrom C, Debridge L. Routine autotranplantation of at least one parathyroid gland during total thyroidectomy may reduce permanent hypoparathyroidism to zero. Aust N Z J Surg 1999;69:794-7. [Crossref] [PubMed]
- Olson JA Jr, DeBenedeti MK, Baumann DS, et al. Parathyroid autotransplantation during thyroidectomy. Results of a long term follow up. Ann Surg 1996;223:472-8. [Crossref] [PubMed]
- Lo CY, Lam KY. Parathyroid autotransplantation during thyroidectomy: is frozen section necessary? Arch Surg 1999;134:258-60. [Crossref] [PubMed]
- Ander S, Johansson K, Smeds S. In situ preservation of the parathyroid glands during operations on the thyroid. Eur J Surg 1997;163:33-7. [PubMed]
- Barczyński M, Cichoń S, Konturek A, et al. Applicability of intraoperative parathyroid hormone assay during total thyroidectomy as a guide for the surgeon to selective parathyroid tissue autotransplantation. World J Surg 2008;32:822-8. [Crossref] [PubMed]
- Gauger PG, Reeve TS, Wilkinson M, et al. Routine parathyroid autotransplantation during total thyroidectomy: the influence of technique. Eur J Surg 2000;166:605-9. [Crossref] [PubMed]
- Funahashi H, Satoh Y, Imai T, et al. Our technique of parathyroid autotransplantation in operation for papillary thyroid carcinoma. Surgery 1993;114:92-6. [PubMed]
- Kikumori T, Imai Y, Tanaka Y, et al. Parathyroid autotransplantation with total thyroidectomy for thyroid carcinoma: long-term follow-up of Grafted Parathyroid Function. Surgery 1999;125:504-8. [Crossref] [PubMed]
- Cavallaro G, Iorio O, Centanni M, et al. Parathyroid reimplantation in forearm subcutaneous tissue during thyroidectomy: a simple and effective way to avoid hypoparathyroidism. World J Surg 2015;39:1936-42. [Crossref] [PubMed]
- Shaha AR, Burnett C, Jaffe BM. Parathyroid autotransplantation during thyroid surgery. J Surg Oncol 1991;46:21-4. [Crossref] [PubMed]
- Testini M, Rosato L, Avenia N, et al. The impact of single parathyroid gland autotransplantation during thyroid surgery on postoperative hypoparathyroidism: a multicenter study. Transplant Proc 2007;39:225-30. [Crossref] [PubMed]
- Hichey RC, Samaan NA. Human parathyroid autotransplantation: proved function by radioimmunoassay of plasma parathyroid hormone. Arch Surg 1975;110:892-5. [Crossref] [PubMed]
- Lo CY, Tam SC. Parathyroid autotransplantation during thyroidectomy: documentation of graft function. Arch Surg 2001;136:1381-5. [Crossref] [PubMed]
- D'Avanzo A, Parangi S, Morita E, et al. Hyperparathyroidism after thyroid surgery and autotransplantation of histologically normal parathyroid glands. J Am Coll Surg 2000;190:546-52. [Crossref] [PubMed]
- Conzo G, Della Pietra C, Tartalia E, et al. Long-term function of parathyroid subcutaneous autoimplantation after presumed total parathyroidectomy in the treatment of secondary hyperparathyroidism. A clinical retrospective study. Int J Surg 2014;12:S165-9. [Crossref] [PubMed]
- Hallgrimsson P, Nordenström E, Almquist M. Risk factors for medically treated hypocalcemia after surgery for Graves' disease: a Swedish multicenter study of 1,157 patients. World J Surg 2012;36:1933-42. [Crossref] [PubMed]
- Kirdak T, Dundar HZ, Uysal E, et al. Outcomes of parathyroid autotransplantation during total thyroidectomy: a comparison with age- and sex-matched controls. J Invest Surg 2017;30:201-9. [Crossref] [PubMed]
- Lo CY, Lam KY. Postoperative hypocalcaemia in patients who did or did not undergo parathyroid autotransplantation during thyroidectomy: A comparative study. Surgery 1998;124:1081-6. [Crossref] [PubMed]
- Ahmed N, Aurangzeb M, Muslim M, et al. Routine parathyroid autotransplantation during total thyroidectomy: a procedure with a predictable outcome. J Pak Med Assoc 2013;63:190-3. [PubMed]
- Wei T, Li Z, Jin J, et al. Autotransplantation of inferior parathyroid glands during central neck dissection for papillary thyroid carcinoma: a retrospective cohort study. Int J Surg 2014;12:1286-90. [Crossref] [PubMed]
- Wells SA Jr, Christiansen C. The transplanted parathyroid gland: Evaluation of cryopreservation and other environmental factors which affect its function. Surgery 1974;75:49-55. [PubMed]
- Brennan MF, Brown EM, Spiegel AM, et al. Autotransplantation of cryopreserved parathyroid tissue in man. Ann Surg 1979;189:139-42. [Crossref] [PubMed]
- Guerrero MA. Cryopreservation of parathyroid glands. Int J Endocrinol 2010;2010:829540.
- Cohen MS, Dilley WG, Wells SA Jr, et al. Long-term functionality of cryopreserved parathyroid autografts: a 13-year prospective analysis. Surgery 2005;138:1033-40. [Crossref] [PubMed]
- Borot S, Lapierre V, Carnaille B, et al. Results of cryopreserved parathyroid autografts: a retrospective multicenter study. Surgery 2010;147:529-35. [Crossref] [PubMed]
- Agarwal A, Waghray A, Gupta S, et al. Cryopreservation of parathyroid tissue: an illustrated technique using the cleveland clinic protocol. J Am Coll Surg 2013;216:e1-9. [Crossref] [PubMed]
- Carter WB, Uy K, Ward MD, et al. Parathyroid-induced angiogenesis is VEGF-dependant. Surgery 2000;128:458-64. [Crossref] [PubMed]
- Tołłoczko T, Woźniewicz B, Sawicki A, et al. Allotransplantation of cultured human parathyroid cells: present status and perspectives. Transplant Proc 1997;29:998-1000. [Crossref] [PubMed]
- Tibell A, Rafael E, Wennberg L, et al. Survival of macroencapsulated allogenic parathyroid tissue one year after transplanytation in non-immunosuppressed humans. Cell Transplant 2001;10:591-9. [PubMed]
- Nawrot I, Woźniewicz B, Tołłoczko T, et al. Allotransplantation of cultured parathyroid progenitor cells without immunosuppression: clinical results. Transplantation 2007;83:734-40. [Crossref] [PubMed]
- Cabané P, Gac P, Amat J, et al. Allotransplantation of microencapsulated parathyroid tissue in severe postsurgical hypoparathyroidism: a case report. Transplant Proc 2009;41:3879-83. [Crossref] [PubMed]
- Woods Ignatoski KM, Bingham EL, Frome LK, et al. Differentiation of precursors into parathyroid-like cells for treatment of hypoparathyroidism. Surgery 2010;148:1186-9. [Crossref] [PubMed]
- Bingham EL, Cheng SP, Woods Ignatoski KM, et al. Differentiation of human embryonic stem cells to a Parathyroid-like phenotype. Stem Cells Dev 2009;18:1071-80. [Crossref] [PubMed]
- Park YS, Kim HS, Jin YM. Differentiated tonsil-derived mesenchymal stem cells embedded in Matrigel restore parathyroid cell functions in rats with parathyroidectomy. Biomaterials 2015;65:140-52. [Crossref] [PubMed]
- Park YS, Hwang JY, Jun Y, et al. Scaffold-free parathyroid tissue engineering using tonsil-derived mesenchymal stem cells. Acta Biomater 2016;35:215-27. [Crossref] [PubMed]
- Kim ES, Keating GM. Recombinant human parathyroid hormone (1-84): A Review in Hypoparathyroidism. Drugs 2015;75:1293-303. [Crossref] [PubMed]
- Jolette J, Wilker CE, Smith SY, et al. Defining a noncarcinogenic dose of recombinant human parathyroid hormone 1-84 in a 2-year study in Fischer 344 rate. Toxicol Pathol 2006;34:929-40. [Crossref] [PubMed]
- Marcucci G, Della Pepa G, Brandi ML. Drug safety evaluation of parathyroid hormone for hypocalcemia in patients with hypoparathyroidism. Expert Opin Drug Saf 2017;16:617-25. [Crossref] [PubMed]