Gasless, transaxillary robotic neck dissection: the technique and evidence
Introduction
Robotic surgery in various surgical fields has been representative of the new horizon of minimally invasive surgery. Its mechanical dexterities were enough to overcome the limitations of laparo-endoscopic surgery, and it has also shown additional superiorities over the other minimally invasive surgeries (1). The main attributed benefits of the robotic surgery can be described as four-fold: (I) tremor-free, stabilized movement of instrument; (II) sophisticated movements with 7-degree freedom by articulating instrument; (III) 3-dimensional, magnified endoscopic view; (IV) optimized ergonomics for the surgeon during the operations (1,2).
For thyroid surgeries, traditional open thyroidectomy has been the gold standard treatment for the surgical thyroid diseases since the era of Theodor Kocher (3). The open method provides direct exposure to the thyroid gland, and subsequently enables the surgeon to perform a safe and quick operation. However, this procedure leaves a conspicuous scar on the exposed part of the patient’s neck a majority of the time. Rapid innovation of technology facilitated new invention of surgical instruments and techniques, and also provided remote-site access thyroidectomy for avoiding obtrusive neck scars (4-7). In 2007, a cutting edge method for thyroidectomy was first introduced using a surgical robotic system developed by Kang et al., (8) and various access points using robotic instruments have been introduced since then, including transaxillary, breast, or both, and retroauricular approach (7-10). Each approach has its own benefits and pitfalls according to the surgeon’s experiences and preference, so no one can definitely argue the supremacy of any one method. However, using extracervical approaches usually do require greater surgical dissection than the conventional open method. Consequently, remote access thyroidectomy could not gain as much world-wide acceptance as the minimally invasive thyroidectomy, in spite of the remote procedure’s esthetic superiority (11).
Meanwhile, the meaning of remote access approach in neck dissection (ND) is somewhat different from that of remote access thyroidectomy. Remote access ND can be considered as a true adoption of the minimally invasive technique (12).
Conventional open ND is considered as the most secure and effective surgical treatment for lateral neck lymph node (LN) clearance, and radical surgical excision and elongated incision on the neck are required. Considering the large dissection area and marked incision scars, remote access ND have been implemented for the replacement of open procedure (13-16). The addition of the surgical robotic system also makes these approaches technically less challenging and improves patient outcomes (12,13). The high-skilled robotic technology capacitates more accurate and elaborate dissection during the lengthy and complicated procedure of ND. Still, among the remote access approaches for ND, the axillary approach appears to be the most preferred method, as it requires the least amount of dissection, and allows for short operation times while leaving the patient’s neck without visible scars (12-16). Over the last few years, the technical practicability, safety, and functional benefits of robotic ND using transaxillary approach have been serially reported, and the oncologic outcome of this technique was shown to be comparable to the open procedure (12-16).
In this chapter, the author will describe the detailed techniques for transaxillary robotic ND, as well as provide evidence for its viability for managing well-differentiated thyroid cancer with lateral neck LN metastasis (LNM).
Surgical technique
Patient preparation
The patient can be enrolled in such cases as well-differentiated thyroid cancer with LNM by preoperative fine needle aspiration biopsy (FNAB). Comprehensive ultrasonography (US) and computed tomography (CT) of the neck can also be used to evaluate the status of a disease’s spread.
The function of the robotic surgery for the treatment of thyroid cancer with LNM remains still disputable. For skillful surgeons, this method can be performed in the selected cases, but its act in cases of locally advanced cancer is doubtful, and thus, robotic ND is evidently contraindicated in the following conditions: (I) obvious tumor invasion to an abutting organ [recurrent laryngeal nerve (RLN), trachea, major vessels, or esophagus]; (II) LNM on the substernal or the infraclavicular area; or (III) peri-nodal tissue infiltration of tumor at the metastatic LN.
Positioning
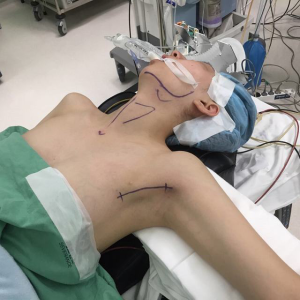
Under general anesthesia, a patient is placed in a comfortable semi recumbent position with a soft pillow under the shoulder. The neck is lightly extended with the face turned to the contralateral side from the lesion. The lesion-side arm is spread out and abducted about 80 degrees from the body (for the complete exposure of the axillar and lateral neck areas) (Figure 1).
Development of working space
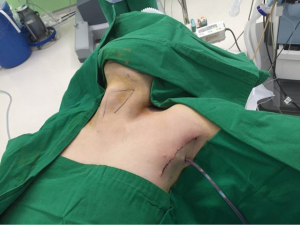
A 10–12 cm curved type skin incision is placed in the axilla along with the lateral border of the pectoralis major muscle. During the design of skin incision, surgeon should always check whether the incision is completely hidden in the natural position. A subcutaneous skin flap is created above the anterior surface of the pectoralis major muscle from axilla to the clavicle. After crossing the clavicle, a further flap is made in a subplatysmal plane. The flap dissection proceeds medially to the bifurcation of two branches of sterno-cleido-mastoid muscle (SCM). The landmark of lateral border of the flap is trapezius muscle. The track of spinal accessory nerve is traced carefully until it passes undersurface of the SCM muscle. The proximal part of external jugular vein is ligated at the crossing point of the lateral margin of SCM. When the subplatysmal skin flap reaches the Erb’s point, the dissection continues under the SCM muscle and extends to the submandibular gland and the posterior belly of digastric muscle. The superior belly of omohyoid muscle is cut at the level of thyroid cartilage, and internal jugular vein (IJV) is identified and carefully separated from the lateral border of strap muscles. Thyroid gland is detached from the strap muscles and completely exposed. After flap dissection, the patient’s face looks forward (neutral position) for bilateral total thyroidectomy. Chung’s retractor, a long and wide retractor blade for the use of ND, is inserted through the axillary incision, and raises the skin flap including SCM and strap muscles for operative field. The entire thyroid gland and lateral neck area are fully exposed by this external retractor system.
Preparation of robotic instruments
The patient cart is docked on the opposite side of the patient’s skin incision, and operating table is slightly rotated to the direction of the patient cart to obtain the linear alignment of the robotic camera arm, the patient’s axillary skin incision and the anterior neck.
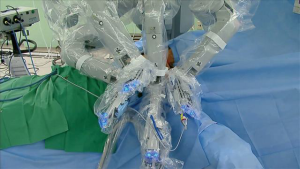
All the four robotic arms are inserted through an axillary incision. To prevent collision between robotic arms, the proper manners of positioning the robotic arms with optimal angles and inter-arm distances are necessary.
For the Rt. side approach, a 30° dual channel endoscope (Intuitive Inc.) with down view is equipped with a 12 mm trocar in the center of the axillary incision. The ProGrasp forceps in an 8 mm trocar is set at the right side of camera, positioned parallel with the retractor blade. Moreover, the ProGrasp forceps should be located as near as possible to the elevated skin flap. The Maryland dissector in a 5 mm trocar gets to the left of the camera (at the left edge of the incision), and the Harmonic curved shears in a 5 mm trocar is positioned at the right side of the camera (at the right edge of the incision). Instruments should be inserted upward direction, and not collide with one another (Figure 2).
Robotic total thyroidectomy with central compartment neck dissection (CCND)
The Harmonic curved shears is used for dissection and the vessel ligation. The thyroid upper pole is pulled toward the medio-inferior direction with the ProGrasp forceps, and the superior thyroidal vessels are identified and individually ligated by Harmonic curved shears. The ProGrasp forceps can provide a stable traction to the upper pole, and constantly changes its position as the dissection proceeds. The superior parathyroid gland is discovered and preserved when the thyroid gland is detached from the cricothyroid muscle. The procedure is performed precisely to prevent the injury of the RLN insertion site. The surgery proceeds to CCND after upper pole dissection. The RLN should be distinguished and tracked its whole running course along with CCND, the thyroid can be separated from the trachea gradually. In the Berry ligament region, cautious dissection is required to avoid direct or indirect thermal injury of the RLN by the Harmonic curved shears. After lobectomy of right thyroid gland, contralateral lobe of the thyroid is also removed in the subcapsular dissection manner, while maintaining the parathyroid glands and the RLN. ND procedure is followed after the bilateral total thyroidectomy.
Robotic ND
The usual surgical extent of ND in differentiated thyroid cancer with LNM contains sublevels IIA, III, IV, and VB, which is applied to both open and robotic ND. After total thyroidectomy with CCND, lateral ND starts with sublevel III&IV first. The IJV is lifted up to the medial direction with the ProGrasp forceps, soft tissues and LNs are drawn with the Maryland dissector and carefully dissected from the anterior surface of the IJV to the posterior aspect of IJV until the common carotid artery and vagus nerve are exposed. Gentle, wiping motions of the Harmonic curved shears can build a proper dissection plane and recognize vascular structures correctly. Skeletonizing process of the IJV progresses downward from the upper level III to level IV area. After medial detachment of the LNs, packets of LNs are then pulled upward. LNs are carefully dissected from the junction of the IJV and subclavian vein, not to injure the thoracic duct. Surgeons may encounter difficulties especially for the right side ND, as the prominent clavicle may interfere with the straight Harmonic in reaching the deepest spot of level IV. In this situation, the remote center in the Harmonic’s arm should be placed higher than a previous position, and the inlet angle of the Harmonic should be increased. In spite of the adjustment of the remote center of Harmonic, this trouble is not cleared, then, the next solution is switching the robotic arms of Harmonic with the Maryland dissector, and putting the straight Harmonic on the left side of the camera allows to reach the deepest area of level IV without any interference. The transverse cervical artery (a branch of the thyrocervical trunk) usually courses horizontally across the anterior scalene muscle, anterior to the phrenic nerve. Considering this anatomic landmark, the phrenic nerve and transverse cervical artery can be protected from injury or ligation. Dissection goes further along the subclavian vein in a lateral direction. After level IV dissection, the inferior belly of omohyoid muscle is divided at the point where it meets trapezius muscle. The distal part of the external jugular vein (which can join the IJV or subclavian vein) is sealed with Hem-o-lok® Clips near to the subclavian vein and cut. LNs of level V are dissected along with the anterior border of trapezius muscle while tracing the course of the spinal accessory nerve. After finishing level III, IV, and VB dissections, re-docking is required to obtain the optimal operation view for the level II LN. The external retractor is removed, and patient face is turned to the opposite side of ND for the ideal exposure of lateral neck. After then, the retractor is re-inserted toward the submandibular gland. The re-docking procedure is conducted in the same manner as the previous docking. Pulling the specimen tissue inferolaterally, the soft tissues and LNs are separated from the lateral border of the sternohyoid muscle, submandibular gland, anterior surfaces of carotid arteries, and the IJV. Sublevel IIA dissection is performed until it reaches the submandibular gland and the posterior belly of digastric muscle superiorly. After the specimen is extracted, the irrigation of operating field is followed, and fibrin glue is spread around the area of the thoracic duct and minor lymphatics. A closed suction drain (3 mm in size) is placed under the axillary skin incision, and the wound closure is performed cosmetically. The incision scar in the axilla is completely hidden when the arm is in its natural position (Figure 3).
The routine placement of drain after the operation may differ in each situation. However, if the amount of drain is less than 50 mL per day, the drain can be safely pulled out without any risk of seroma.
Evidence
An ND technique in the malignancy was firstly described in 1906 (17). At that time, this operation referred to the removal of all lymphatic and non-lymphatic structures between the platysma and the prevertebral fascia in the lateral neck, except for common carotid artery and vital motor nerves (17). Since then, ND method has been continuously changed to preserve functioning structures more and reduce surgical morbidity meanwhile keeping the oncologic safety (15). Since 1980, the modified radical neck dissection (MRND)—preserving IJV, SCM, and spinal accessory nerve, has gained wide acceptances as a substitutes for the traditional ND in terms of reduced functional disability for the patients (18). In well differentiated thyroid cancer (WDTC), the principle of surgical technique was not so different from that of other malignancies except level I dissection. Because the cases with level I or level IIb metastasis from WDTC were so rare, routine extents of ND for WDTC has included sublevel IIa, III, IV, V, and VI. Even though, the surgical extents for the ND in WDTC has been decreased, the incision and area of subplatysmal skin flap were not changed much compared to that of traditional ND (12,13,18). Many of the people who had been performed ND suffered from the frustrating incision scar and neck discomfort by the extensive flap adhesion in spite of successful removal of the diseased tissues. Some of the innovative surgeons have continuously tried to apply the minimally invasive techniques to the ND in WDTC, and this could be much evolved by the incorporation of the surgical robotic system (12,13,15). The first report of robotic ND was in 2010, and was described by Yonsei group from South Korea which has the largest experience for the robotic thyroidectomy (12). Since then, serial reports about the surgical safety, post-operative functional benefits of the patient, and oncologic outcomes of the robotic ND have been introduced (12-16).
Surgical safety and outcome
For the reasonable adoption of the new surgical skill, the safety and feasibility of it should absolutely be clarified. For the surgical safety, it is a common agreement that the surgical outcome and complication rate of the new technique should be better than, or at least similar to that of previous technique.
Kang et al. have firstly described the detailed technique of this new approach with the outcomes in 33 WDTC patients. All the patients have been safely performed bilateral total thyroidectomy with ipsilateral CCND and MRND together without any neck scar (12). They showed the mean operating time as 280.8±40.6 min and the mean number of retrieved LNs as 33.0±11.6 without any serious postoperative complications. Another study from the same group which was designed to compare the surgical outcomes between robotic and open ND revealed a little longer operation time in robotic ND (277.4±43.2 in robotic vs. 218.2±43.8 min in open; P<0.001) (13). However, there were no statistical differences in the numbers of retrieved LNs and complication rate between the two groups. Furthermore, the mean postoperative hospital stay was shorter in the robotic group (6.0±2.5 in robotic vs. 8.0±5.2 days in open; P=0.008) (13). Lee et al. also reported the comparative data between robotic and open ND, and they indicated a longer operation time in robotic than open group (271.8±50.2 in robotic vs. 208.9±56.3 in open; P<0.001), but showed similar results in the retrieved LNs, hospital stay, and complication rate (14). All the reported study for the robotic ND showed similar surgical outcome with open ND except the operation time, and there was no noticeable complication such as permanent hypocalcemia or vocal cord paralysis, post op. bleeding, motor nerve injury, or major chyle leakage in the robotic group (12-14,16).
The robotic ND is actually a three-stage procedure that involves making a working space, preparation of robotic instruments, and the literal thyroidectomy and ND. Different from the thoracic or abdominal procedure, there is no free space in the neck, so additional time for the working space creation is always necessary in the robotic ND. The point that data in this study included their initial experience of robotic technique can be also another probable factor for the prolonged operation time in the robotic group (12-14).
Oncologic safety
To showing the oncologic safety of new procedure is really essential for the surgical treatment of malignant disease. WDTC usually managed by surgical resection and followed by radioactive iodine therapy according to the risk stratification. The survey for the recurrence is usually performed by regular neck US, radioactive (RI) scan, and serum thyroglobulin (Tg) level [with thyroid stimulating hormone (TSH) stimulated or suppressed].
Kang et al. described the oncologic safety of robotic ND after 1-year follow-up in their study. According to the data, there was no evidence of recurrence in neck US and RI scan in both robotic and open ND group (13). Serum Tg levels (TSH suppressed) were measured at 6 months interval postoperatively and found to be maintained at low levels. Mean Tg levels did not differ significantly in the two groups (0.54±0.98 in the open vs. 0.64±1.63 in the robotic; P=0.684). Among 165 patients, only three in robotic and seven in open group had a serum Tg >1 ng/mL (Tg levels were 4.59±4.54 and 3.41±2.40, respectively) (13).
Lee et al. also reported the detailed outcomes of oncologic safety in robotic ND (14). They emphasized on the the numbers of dissected LNs and the completeness of thyroid resection for the oncologic safety. They showed that the total numbers of retrieved LNs were similar for robotic and open procedures (38.0±14.1 vs. 37.9±16.8, P=0.5120). After mean follow-up of 8.4 months (range, 6–12 months), no patient in either group showed tumor recurrence on neck US and other imaging modalities. Among the 128 patients, 4 in open group (6.1%) and 4 in the robotic group (6.4%) had serum Tg >1 ng/mL, however, none showed abnormal foci of increased radioiodine uptake in RI scan. Moreover, the mean Tg levels (TSH suppressed) did not differ significantly in the two groups (0.61±0.49 in robotic vs. 0.51±0.48 in open; P=0.7411) (14).
Kim et al. assessed the long-term (more than 5 years) oncologic outcomes of robotic ND different from the other study (16). In their study, the median follow-up period was 66.0 months (range, 60–90 months). They also compared the oncologic outcomes between robotic and open ND group, and to decrease the risk of confounding factors (age, stage and tumor size), an exact 1:3 matching analysis was performed.
In this study, no significant difference were found in the number of retrieved LNs (P=0.102) and postoperative ablation success rates (P=0.864) between the two groups.
During the follow-up periods, serum Tg level (TSH-suppressed) after 5 years (0.7±1.5 in robotic vs. 2.4±14.1 ng/mL in open; P=0.471) and recurrence rates could not show any differences between the two groups [1/41 (2.4%) in robotic vs. 3/102 (2.9%) in open; P=0.864], neither. The 5-year median recurrence free survival was similar between the two groups (60.8±12.0 in robotic vs. 67.1±15.5 months in open; P=0.936). There was no disease-specific mortality during that period (16).
Post-operative quality of life (QoL) in patient’s perspective
In recent years, with the introduction of various surgical approaches for minimally invasive technique to thyroid, increasing attention beyond the surgical and oncologic safety has been given to the advantage for patient’s perspective after the operation, such as postoperative pain, sensory changes, voice and swallowing functions, and cosmetic satisfaction. It is already well-known that remote access approach for thyroid provides better cosmetic satisfaction and less postoperative neck paresthesia and discomfort than the open procedure (19-21).
In case of robotic approach, there were also some reports about faster recovery of voice and swallowing function than open thyroidectomy (20,21).
Considering the wide extent of surgical dissection in robotic ND, the study by Lee et al. is well highlighting the issues about excellence in the patient’s QoL after robotic ND (14). The study directly compared the effects of robotic and open ND on postoperative QoL, including shoulder disability in WDTC patients. They used the validated, reliable self-assessment questionnaires evaluating pain score of the surgical scar, sensory change, cosmetic satisfaction, voice and swallow difficulties, and the functional change and pain of the ipsilateral shoulder.
The study showed markedly reduced sensory change around the neck and anterior chest (hyperesthesia or paresthesia) and swallow discomfort, as well as extreme satisfaction with the cosmesis in the robotic group (Table 1). They also showed slightly decreased pain on the operative scar and voice change in robotic group, yet, there was no statistical difference. There was no significant difference in the shoulder impairment and pain between the two groups, neither (14).

Full table
Conclusions
With the dexterities of surgical robotic system, the cutting-edge technique for ND using transaxillary approach have introduced and serially showed satisfactory evidences for this technique. Robotic ND showed a similar surgical outcome and oncologic safety for the management of WDTC with lateral neck metastasis without any serious complication. Furthermore, this technique could give additional advantages for QoL, in patient’s perspective, such as decreased sensory change in the neck and chest, reduced swallow difficulty, and excellent cosmesis.
Through these evidences, the authors who have the largest volumes of robotic ND can insist that this approach allows compartment-oriented LN dissection in the lateral neck without any injury of major vessels, or nerves.
With further research and evidences for robotic ND, this technique might be an alternative means of surgery in WDTC with LNM.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Peña González JA, Pascual Queralt M, Salvador Bayarri JT, et al. Evolution of open versus laparoscopic/robotic surgery: 10 years of changes in urology. Actas Urol Esp 2010;34:223-31. [PubMed]
- Herron DM, Marohn M. SAGES-MIRA Robotic Surgery Consensus Group. A consensus document on robotic surgery. Surg Endosc 2008;22:313-25. [Crossref] [PubMed]
- Seybt MW, Terris DJ. Minimally invasive thyroid and parathyroid surgery: where are we now and where are we going? Otolaryngol Clin North Am 2010;43:375-80. [Crossref] [PubMed]
- Yoon JH, Park CH, Chung WY. Gasless endoscopic thyroidectomy via an axillary approach: experience of 30 cases. Surg Laparosc Endosc Percutan Tech 2006;16:226-31. [Crossref] [PubMed]
- Ikeda Y, Takami H, Niimi M, et al. Endoscopic thyroidectomy and parathyroidectomy by the axillary approach. A preliminary report. Surg Endosc 2002;16:92-5. [Crossref] [PubMed]
- Miccoli P, Elisei R, Materazzi G, et al. Minimally invasive video-assisted thyroidectomy for papillary carcinoma: a prospective study of its completeness. Surgery 2002;132:1070-3; discussion 1073-4. [Crossref] [PubMed]
- Lee KE, Rao J, Youn YK. Endoscopic thyroidectomy with the da Vinci robot system using the bilateral axillary breast approach (BABA) technique: our initial experience. Surg Laparosc Endosc Percutan Tech 2009;19:e71-5. [Crossref] [PubMed]
- Kang SW, Jeong JJ, Yun JS, et al. Robot-assisted endoscopic surgery for thyroid cancer: experience with the first 100 patients. Surg Endosc 2009;23:2399-406. [Crossref] [PubMed]
- Ryu HR, Kang SW, Lee SH, et al. Feasibility and safety of a new robotic thyroidectomy through a gasless, transaxillary single-incision approach. J Am Coll Surg 2010;211:e13-9. [Crossref] [PubMed]
- Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: II. Clinical feasibility and safety. Laryngoscope 2011;121:1636-41. [Crossref] [PubMed]
- Terris DJ, Singer MC. Robotic facelift thyroidectomy: Facilitating remote access surgery. Head Neck 2012;34:746-7. [Crossref] [PubMed]
- Kang SW, Lee SH, Ryu HR, et al. Initial experience with robot-assisted modified radical neck dissection for the management of thyroid carcinoma with lateral neck node metastasis. Surgery 2010;148:1214-21. [Crossref] [PubMed]
- Kang SW, Lee S, Park JH, et al. A comparative study of the surgical outcomes of robotic and conventional open modified radical neck dissection for papillary thyroid carcinoma with lateral neck node metastasis. Surg Endosc 2012;26:3251-7. [Crossref] [PubMed]
- Lee J, Kwon IS, Bae EH, et al. Comparative analysis of oncological outcomes and quality of life after robotic versus conventional open thyroidectomy with modified radical neck dissection in patients with papillary thyroid carcinoma and lateral neck node metastases. J Clin Endocrinol Metab 2013;98:2701-8. [Crossref] [PubMed]
- Lee J, Chung WY. Current status of robotic thyroidectomy and neck dissection using a gasless transaxillary approach. Curr Opin Oncol 2012;24:7-15. [Crossref] [PubMed]
- Kim MJ, Lee J, Lee SG, et al. Transaxillary robotic modified radical neck dissection: a 5-year assessment of operative and oncologic outcomes. Surg Endosc 2017;31:1599-606. [Crossref] [PubMed]
- Crile GW. On the surgical treatment of cancer of the head and neck. With a summary of one hundred and five patients. Trans South Surg Gynecol Assoc 1905;18:109-27.
- Kowalski LP, Sanabria A. Elective neck dissection in oral carcinoma: a critical review of the evidence. Acta Otorhinolaryngol Ital 2007;27:113-7. [PubMed]
- Lee J, Nah KY, Kim RM, et al. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc 2010;24:3186-94. [Crossref] [PubMed]
- Tae K, Ji YB, Jeong JH, et al. Robotic thyroidectomy by a gasless unilateral axillo-breast or axillary approach: our early experiences. Surg Endosc 2011;25:221-8. [Crossref] [PubMed]
- Lee S, Ryu HR, Park JH, et al. Early surgical outcomes comparison between robotic and conventional open thyroid surgery for papillary thyroid microcarcinoma. Surgery 2012;151:724-30. [Crossref] [PubMed]