Nipple sparing mastectomy techniques: a literature review and an inframammary technique
Introduction
Less aggressive surgical management of breast cancer patients has made major advances over the last 3 decades with the rebuke of the Halstedian concept to acceptance of a more systemic approach to the management of breast cancer patients. Surgery has become more conservative starting with Bernie Fisher’s proven concept of breast conservation in the 1980’s and then the advent of sentinel lymph node (SLN) evaluations and skin sparing mastectomies in the 1990’s. With studies proving the low risk of nipple areolar complex (NAC) involvement in select cancer patients (1-3) and the new genetic era of breast cancer risk, it was only natural that nipple sparing techniques would be developed in the late 1990’s and at the turn of the century. In 1999, Lynn Hartmann’s paper (4) in the NEJM showed the benefit for prophylactic, or better termed risk reducing nipple sparing mastectomy (NSM), in high risk patients with a 90% reduction in breast cancer development. This first started the movement towards the nipple sparing approach. Meijers-Heijboer’s later study (5) in the NEJM along with other publications (6-9) then proved the advantages of risk reducing mastectomy in BRCA+ patients. These cancer reduction benefits along with the improved aesthetic outcome from optimal breast contouring with minimal scarring and improved patient satisfaction led to the NSM’s quick acceptance and implementation into breast surgery practices.
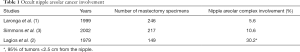
The use of the NSM in cancer patients is more controversial and though it has become standard practice in many early stage cancer patients, who require or request a mastectomy, there are no controlled clinical trials evaluating its effectiveness. The initial use of the nipple sparing approach was spurred by evidence showing a low risk of NAC involvement in the pathological mastectomy specimens of smaller cancers (less than 2 cm) which are node negative, more peripherally located (>2 cm from the nipple), and localized (1-3) (Table 1). This led various institutions to begin performing NSMs in this well-defined lower risk group of breast cancer patients with good short-term cancer outcomes (10-13). This has now progressed to the expansion of eligibility criteria with some institutions advocating for its use in higher risk patients with larger tumors, tumors close to the nipple, or even in patients with more aggressive cancers after neoadjuvant chemotherapy (14). Its utilization has also increased with the greater use of mastectomy and contralateral prophylactic mastectomy (15,16) especially in the younger aged breast cancer population (17,18). To date, only one meta-analysis (19) has critically analyzed overall survival (OS), disease-free survival (DFS), and local recurrence (LR) in cancer patients who underwent NSM showing no significant differences compared to women undergoing modified radical mastectomy (MRM) or skin sparing mastectomy (SSM). This meta-analysis is limited in that it focuses mainly on short-term follow-up studies in earlier stage cancer patients. The American Society of Breast Surgeons (ASBS) currently has an on-going registry trial tracking NSM patients throughout the United States to help better define patient outcomes and eligibility criteria in the future.
Full table
In this review, we have attempted to search for the best studies of the NSM approach to determine the optimum technique with the lowest complication rates, trying to identify the technical causes of the most common complications and the best methods to avoid them. We focused on studies evaluating surgeon specific variables as well as patient variables which help to reduce post-operative complications. We only briefly mention the clinical oncologic indications for the procedure and cancer specific outcome data as well as specific reconstruction technique unless it had some bearing on overall complication or outcome since these topics are being described elsewhere in this journal edition.
Methods
We researched articles using MEDLINE and PubMed using the MeSH headings for “nipple sparing or total SSM, technique, complications, outcomes, or satisfaction”. We performed a world-wide search of all English language journals. We chose relevant articles which focused mainly on technical factors including complications and patient satisfaction.
Results
SLN biopsy in prophylactic surgery
The use of SLN biopsy in prophylactic surgery has been studied in at least three separate institutional studies showing the frequency of occult cancer in prophylactic mastectomy patients to be less than 10% with the majority of the occult cancers found to be ductal carcinoma in situ (DCIS) (20-22) (Table 2). The rate of occult invasive cancers, being less than 5%, does not justify the use of routine SLN biopsy for patients undergoing nipple sparing mastectomies in the prophylactic setting.

Full table
Incision placement
Various incision locations have been described for NSMs with individual surgeons or institutions tending to favor certain approaches (23-27). The incisions used most frequently were best evaluated by Endara et al. (26) who analyzed 48 pooled NSM studies from the literature with 41 studies describing the mastectomy incision and 11 studies evaluable for outcomes by incision type which found the most common incision used was a radial approach (46%) followed by the periareolar (27%), and the Inframammary incision (21%). The Endara et al. (26) study also looked at nipple necrosis rates associated with incision placement in the same 11 studies which included 543 procedures and found the lowest rates of nipple necrosis in the incisions involving the least circumference of the nipple (radial incision, 8.83%; inframammary, 9.09%; periareolar/circumareolar, 17.81% and transareolar, 81.82%). Increased risk of nipple areolar necrosis associated with periareolar incisions was also seen in an Italian study by Salgarello et al. (28) where it was seen as an early complication in 4 of the breasts [9.5% (4/42) of total NSMs] with periareolar incisions but in none of the 22 breasts with radial incisions undergoing NSM from 2004 to 2009.
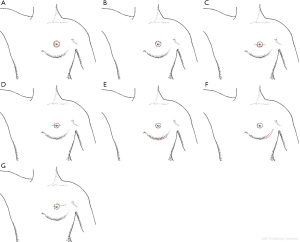
An earlier single institution study which evaluated incision location and nipple necrosis rates was performed at University of California, San Francisco (UC-SF) and published by Wijayanayagam et al. (29) and Garwood et al. (13) and reported two general types of incisions crossing either >30% of the NAC (NAC crossing, mastopexy, nipple free grafts) or <30% of the NAC (inframammary, radial, or lateral/inferolateral) (Figure 1). Patients were divided into 2 cohorts, an earlier group of 64 NSM procedures from 2001 to 2005 where more NAC crossing incisions were performed vs. a later cohort of 106 NSM procedures from 2005 to 2007 where fewer NAC crossing incisions were performed. There was a significant increase in nipple survival rates (80–95%, P=0.003) and decrease in necrotic complications (30–13%, P=0.01) in the later cohort. A further follow-up study by Warren Peled et al. (11) from UC-SF compared their first 100 NSM cases with the following 557 NSM cases and continued to show decreased complications involving both nipple and mastectomy flap necrosis as well as reductions in expander/implant loss which they again attributed to fewer NAC crossing incisions (mainly inframammary or limited superior areolar incisions) as well to reduced use of direct to implant reconstruction and the selective use of acellular dermal matrixes (ADMs) during reconstruction.
Overall cosmetic and satisfaction outcomes as related to incision types have been reported but not statistically analyzed by a few individual studies (23,25,30-33). A retrospective study by Djohan et al. (32) best evaluated cosmesis and patient satisfaction using postop questionnaires of 78 patients as well as independent observer opinions. Seventy-three percent of the patients stated that they would undergo the surgery again. Decreased nipple sensation/arousal was the most common complaint followed by lateral displacement of the nipple. Nipple displacement was felt to be related to the radial incision used for the majority of the NSMs and was related to scar contraction. A separate study by Wagner et al. (30) evaluated cosmetic outcomes of 26 patients who underwent NSM through independent evaluations by two plastic surgeons 6 months after surgery. There was an acceptable (excellent, very good, or good) appearance in the breasts of 73.1% of the patients and in the NAC of 55.8% of the patients. The biggest cosmetic problem was described as lateral displacement of the nipple in 67.4% of patients or lateral displacement of the breast in 50% of the patients and this was felt to be due to the lateral incision placements in the majority (79.6%) of the patients. Other single institution studies have suggested that keeping the incisions in the inframammary (25) or inferolateral (23,34) positions (away from the nipples) does not result in nipple lateralization and also offers better concealment of the incision. Moyer et al. (33) reported on a retrospective database evaluation of NSMs in 26 patients (40 NSMs) performed from 2009 to 2010 where postoperative photographs were evaluated by four reviewers. Circumareolar incisions were associated with a nipple necrosis rate of 75% compared with 33% for radial incisions and 27% for inframammary incisions and were related to worse aesthetic evaluations. Not one of these studies was set up to statistically evaluate the cosmesis and satisfaction rates of NSM independent of the incision type and they are just single institution observations.
Preserving the vasculature
The maintenance of the NAC viability and skin flap perfusion has been studied in past female breast cadaveric studies by van Deventer et al. (35) and O’dey et al. (36). The NAC gets the majority of its blood supply from the internal thoracic vessel with its medial perforators and the lateral thoracic vessel (Table 3). O’dey et al. (36) suggested that medial and lateral based pedicle flaps (superomedial and superolateral) may provide the best blood supply to the nipple which would favor a more inferior incision to preserve these pedicles. If a radial incision is used then a more lateral incision would be favored to help preserve as much of the internal thoracic blood supple coming off the medial flap as possible. A full-thickness glandular dermal skin flap dissection, leaving much of the subdermal fat, was also felt to be beneficial for vascular preservation as opposed to a thinner split-thickness lipo-dermal flap, where more of the subdermal fat is removed.

Full table
Preservation of the NAC
We found no studies that were designed to determine exactly how to handle the thickness of tissue left under the NAC with different surgeons applying different techniques and strategies. Studies have not been specifically controlled to determine if either everting and coring the nipple to remove all the visible ductal tissue (27,29) vs. a more conservative approach of leaving a visible rim of tissue in and around the nipple and areola (24,37) leads to better nipple viability. Petit et al. (38) from Milan reported on follow-up at 5 years of 1,001 NSMs where ELIOT and intraoperative radiation, was applied to the retroareolar residual nipple tissue with excellent results (nipple necrosis rate of 3.5%). Another study from Rusby et al. (39) looked at the microscopic anatomy of the NAC in 7 non-irradiated and 5 irradiated nipples and found that removal of the duct bundle in the center of the nipple and leaving a 2 or 3 mm peripheral rim of subcutaneous tissue around the nipple removed 96% (2 mm) and 87% (3 mm) of the ductal tissue, respectively. The study also found that leaving a 2 or 3 mm peripheral rim of subcutaneous tissue around the nipple retains 50% (2 mm) and 66% (3 mm) of the vascularity of the nipple, respectively and that radiation did not affect the vascular density of the ductal tissue in the nipple. These individual institutional trials and studies give some experimental support to validate the concept of acceptable NAC viability yet good ductal tissue clearance with the technique of leaving small residual rims of retroareolar breast tissue during NSMs.
The use of a “delay phenomenon” by creating a surgical wound to improve the blood supply to the NAC prior to the NSM has also been described with good results (40,41). The procedure involves a periareolar incision to elevate a plane beneath the NAC 1–3 weeks before the planned NSM and thus stimulating improved blood supply to the wounded tissue. Jensen et al. (41) emphasized its use, without any NAC loss, in 20 patients with prior areolar incisions, significant ptosis, or smokers. In lower risk patients, where 360° dermal perfusion could be preserved, however, its use was not felt necessary.
Pathologic assessment of the NAC
Intraoperative pathologic evaluation of the NAC to determine preservation was initially a standard during all NSMs to determine, intraoperatively, if the NAC could be preserved in patients. This has recently been abandoned by some practices (24) due to rare instances of positive biopsies. Its use was also questioned in a poster from the ASBS registry (42) due to the low rates of intraoperative involvement (2/104 NAC biopsies) and 2 nipples removed due to false positive intraoperative results which were read as indeterminate or suspicious and were found to be cancer-free after the nipples were removed intraoperatively.
Tumescence
The use of tumescence as an aid in raising skin flaps to decrease bleeding using lactated ringers solution containing 1% lidocaine and dilute (1:1,000) epinephrine has been previously described for use in mastectomies (43). Tumescence was not shown to be an independent variable affecting post-operative complications including infection, flap necrosis, hematoma, seroma, or epidermolysis in two separate studies of non-NSMs performed by Khavanin et al. (44) and Abbott et al. (45). It has not been well studied in NSM patients and is currently being used as an aid in some institutions to decrease bleeding with sharp (scissor or knife) dissection to avoid electrocautery thermal injury to the skin but should be used with caution because of the temporary vasoconstrictive properties of the epinephrine. I could only find one paper which statistically evaluated tumescence in NSM but solely related to expander reconstruction in 966 patients undergoing SSM and NSM (46) which found that tumescence was an independent risk factor associated with increased flap necrosis (12.8% with tumescence vs. 6.7% without) in those patients who had high intraoperative expander fill volumes (>66% maximal fill volume).
Surgeon experience
There is suggestive evidence and good reason to believe that surgical experience plays a role in reducing complications and improving outcomes in NSM but only one study performed by Gould et al. (47) specifically analyzed surgical experience with complications, specifically nipple necrosis rates. In this study, there was no significant reduction in nipple loss rates with surgeon experience of 1–2 cases (15%) vs. 3–10 cases (23%) vs. >10 cases (20%). This study, however, goes against other institutional experiences with larger numbers of cases showing improved complication rates as surgical experience and technique improves (13,24,27,32,34). Garwood et al. (13) in the 2-cohort study showed a significant decrease in necrotic skin complications (30% to 13%) and an improvement in nipple survival (80% to 95%) with a later cohort of NSM patients performed from 2005 to 2007 compared to an earlier cohort from 2001 to 2005. A retrospective study by Colwell et al. (34) of 500 consecutive NSMs from 2007 to 2012 showed a 5-year trend towards inferolateral/inframammary incisions with lower complication rates shown by multivariate analysis [odds ratio (OR), 0.018; 95% CI, 0.00260–0.12089] which helped to modified their subsequent incisions. Crowe et al. showed improvements in NAC viability comparing a 2004 study (48) of 54 NSMs vs. a 2008 paper (27) of 149 NSMs with the later paper using only laterally and the earlier paper using medially placed incisions without NAC loss in their later experience.
Selection factors
Surgical selection criteria have to be considered when performing NSMs. Reviewed studies showed no difference in complication rates (30,47) or patient satisfaction rates (10) when the NSM was performed for cancer (therapeutic) or for prophylactic reasons. Bilateral NSM procedures compared to unilateral procedures, in the study by Wagner et al. (30), showed no increase in complication rates as well. A single institution retrospective review from 2003 to 2011 by Gould et al. (47) comparing 113 NSM cases to a matched group of 120 SSM controls (28% vs. 27%) also found no significant differences in overall complication rates.
Patient specific selection criteria, however, does affect the outcome of NSMs. The effect of these patient specific variables on complication rates have not been well studied but has been best described by Gould et al. (47) in a series of 73 women who underwent NSMs from 2003 to 2011. BMI, diabetes mellitus, hypertension (HTN), and smoking showed a nonsignificant trend towards worsening complications of nipple necrosis. Large bra cup size (C or larger) was the only statistically significant patient factor with a higher nipple necrosis rate of 34% compared to only 6% with B or smaller cup sizes. Djohan et al. (32), in his patient satisfaction survey of 78 NSM patients, also correlated lower patient satisfactions and increased complications with larger breasts and increased BMI. BMI, smoking and preoperative radiation were associated with higher total complication rates in the study by Colwell et al. (34) which evaluated 500 NSMs from 2007 to 2012. In the 2-cohort study by Garwood et al. (13) smoking was also a statistically relevant independent variable associated with increased skin/nipple necrosis rates.
Reconstruction methods
Reconstruction method has also been linked to complications associated with NSM. Endara et al. (26) reported a pooled analysis study which compared 5 2-stage implant reconstruction studies vs. 5 direct to implant reconstruction studies vs. 2 autologous reconstruction studies and resulted in complication rates of 52.8%, 16.7%, and 23.7% with nipple necrosis rates of 4.5%, 4.1%, and 17.3%, respectively. Gould et al. (47) showed no significant effect of reconstruction type on nipple necrosis complications in the 113 NSM cases evaluated but there was a trend toward higher nipple necrosis rates with autologous vs. either 2-stage or direct to implant reconstructions (40% vs. 18.5%, P=0.23). In comparing the 2-stage vs. the direct to implant reconstruction, Colwell et al. (34) did not see a difference in complication rates in the 500 NSMs evaluated however, with experience they have developed selection criteria using the 2-stage reconstruction more selectively in patients at higher risk of nipple or skin flap necrosis (e.g., smokers, higher BMI). In Garwood et al.’s (13) 2-cohort study, a higher rate of necrotic skin complications resulted with immediate implant reconstruction in their initial cohort of NSM patients causing them to switch to 2-stage reconstructions in their later cohort of NSM patients with a reduction in the complication rate. In that study, autologous reconstruction still accounted for the highest skin necrosis complication rates of all of the reconstruction techniques (37% autologous complication rate vs. 18% for direct to implant vs. 7% for 2-stage reconstruction).
The inframammary nipple sparing technique (Figure 2)
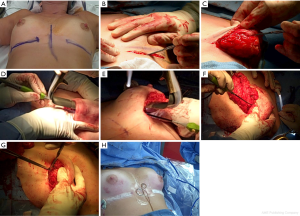
Our initial experience and technique of NSM from 1988 to 2007 including 67 patients has been previously described (49) with the majority of our procedures performed prophylactically (79%). We have now performed over 600 NSMs with 95% performed by one breast surgeon (AYA) and one plastic surgeon (CAS). Since our initial paper, we have seen a significant increase in the numbers of NSMs performed for cancer as acceptance and eligibility has expanded.
Our technique involves a coordinated approach with our reconstructive surgeons especially as it relates to patient factors including comorbidities and breast size which have been shown to have significant impacts on outcomes. If large breast size or significant ptosis is felt to affect outcome, we have adopted Dr. Spear’s described technique (50) of a staged reduction mastopexy and delayed NSM, if the clinical situation allows. Previous augmentation, previous breast surgery, and even prior radiation have not been absolute contraindications for the procedure in our practice.
We perform MRI preoperatively in all our patients who are felt to be NSM candidates to rule out possible mammographic and ultrasound occult tumors in high-risk patients choosing risk reducing NSM and to determine eligibility of NSM in early breast cancer patients. We will allow multifocality as long as the tumors are >2 cm from the nipple and the disease is contained to one quadrant.
We begin by marking out symmetrical inframammary incisions with our plastic surgeon. The incision length varies from 8–11 cm depending on the breast anatomy in order to obtain appropriate exposure. Larger incisions can be required in some of the larger breasts or in patients with dense breast tissue where the skin is fairly taught. We have performed longer, more medially base inframammary incisions to allow for internal mammary artery access for deep inferior epigastric perforator (DIEP) flap reconstructions in selected cases. The incisions are planned to lie in the new inframammary fold as determined preoperatively by the reconstructive surgeons (usually about 7.5–8.0 cm from the nipple). Once the incision is made, the superior skin flap is everted with the non-dominant hand and the breast tissue retracted inferiorly with two Adair clamps. The plane between the subcutaneous fat and the glandular tissue is developed to preserve the dermal blood supply. We have used sharp knife dissection but typically use the Peak PlasmaBladeTM radiofrequency device (Medtronic, Palo Alto, CA, USA). We do not use tumescence in order to prevent any short-term effect of vasoconstriction related to the epinephrine. Care must be taken while raising the initial inframammary flap below the nipple because this is the most ischemic part of the skin flap and surgeons often strip too much of the subcutaneous fat in this area exposing the dermal vessels to injury. The breast tissue is freed using this skin eversion technique past the posterior aspect of the NAC with only a small approximately 2–3 mm thick rim of breast tissue visibly left under the NAC. The nipple itself is not inverted and cored, in our technique, to prevent increased risks of nipple ischemia. Once the skin flap is developed to a plane where direct visualization is impossible by the skin eversion technique, lighted fiberoptic retraction (InvuityTM, Invuity, San Francisco, CA or LightMatTM, Cura Surgical, Geneva, IL, USA) is used to visualize the plane superiorly up to the infraclavicular region, medially to the sternum, and laterally to the latissimus. Care is taken to preserve the intercostal perforators coming medially off the sternum which can supply a significant vascular supply to the skin flaps. Finally, electrocautery is used to remove the breast off the pectoralis major muscle. The axillary tail of the breast is the highest and most difficult to visualize, so this region is typically dissected last so as to use the countertraction of the breast to better visualize and remove these last breast/skin attachments. A retroareolar biopsy is then taken as a shave biopsy underneath the nipple and sent for intraoperative frozen section analysis and if positive for cancer, the NAC is removed through a separate horizontal elliptical incision during the same procedure. The breast tissue is often weighed by the plastic surgeons to help them determine the subsequent reconstruction volumes. The skin flaps are then visualized and trimmed, if necessary, to remove any residual breast tissue and to ensure even flaps.
SLN biopsies are performed only in therapeutic cancer cases through a small separate axillary incision which can also be used to help visualize and assist in the removal of the axillary breast tissue. Tc99 is used alone without using either methylene blue or isosulfan blue dye to prevent the vasoconstriction associated with the blue dyes and potential effects on nipple viability. We use intraoperative skin angiography (SPY EliteTM, Novadaq, Ontario, Canada) in most of our cases at two separate time points, after the mastectomy and after the reconstruction to evaluate the skin flaps. There is limited data to quantify the absolute risk of skin flap necrosis with this device but we have found it helpful to identify possible areas of concern which we will monitor more closely. Since we use intraoperative skin angiography, we do not use tumescence since it causes significant vasoconstriction and poor visualization of the dermal vessels during angiography, making any predictions of skin/nipple viability nearly impossible. An upper body warmer (Bair HuggerTM, 3M, St. Paul, MN, USA) is kept on our patients for the first 24 hours to help prevent vasoconstriction. For post-operative pain control, we have used a variety of methods including Marcaine pumps (On-Q, Halyard Health, Irvine, CA, USA) placed subcutaneously under the skin flaps as well as pre-operative pectoral nerve blocks and more recently liposomal bupivacaine (ExparelTM, Pacira Pharmaceuticals, Parsippany, NJ, USA) injected into the pectoral muscle just prior to reconstruction.
Discussion
The concept and technique of NSM was originally described by Freeman in the 1960’s (51) but it was only described for high-risk patients since it was not accepted for cancer patients as the dogma of radical extirpation of cancer persisted up until the 80’s. As the BRCA gene was identified by Mary Claire King in the 1990’s, the NSM made a resurgence as an accepted procedure for high-risk patients with its reintroduction by Hartmann et al. (4) and later benefits reported in the BRCA population (5,6,8,52).
The beginning of the century then saw the use of NSMs in cancer patients with some encouraging initial follow-up results (10,19,26,38,53,54) as measured by short term OS and LR rates. Of the papers with reasonable follow-up data, Endara et al. (26) reported locoregional recurrence rates (LRR) of 1.8% and distant metastasis rates (DM) of 2.2% in 28 pooled studies but follow-up was short ranging from 0.2–210 months and the tumor types and characteristics were not independently reported in the study. A meta-analysis reported by De La Cruz et al. (19) looked at eight studies comparing NSM with MRM/SSM with no statistically significant differences between the treatment groups in terms of use of adjuvant or neoadjuvant chemotherapy, use of adjuvant radiation, estrogen receptor (ER) or progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2)/neu status, lymph node status or tumor sizes. Five of the 8 studies compared DFS with a 9.6% risk benefit for NSM, 5 studies compared OS with a 3.4% risk benefit for NSM, and 8 studies evaluated LR with a 0.4% benefit for NSM. None of these benefits for DFS, OS, or LR were statistically significant, however. Follow-up times ranged from 25 to 101 months. Gerber et al. (54) compared LRR, DM, and breast cancer specific death rates in a series of 238 patients from 1994–2000 who were candidates for MRM with tumors greater than 2 cm from the nipple and no skin involvement, and were then offered either SSM, NSM, or MRM. Forty-eight patients underwent SSM, 60 patients underwent NSM, and 130 patients underwent MRM and no significant differences resulted after a mean follow-up of 101 months (LRR: 10.4% SSM, 11.7% NSM, 11.5% MRM, P=0.974; DM: 25% SSM, 23.3% NSM, 26.2% MRM, P=0.916; breast cancer specific death: 20.8% SSM, 21.7% NSM, 21.5% MRM, P=0.993). The largest prospective trial reporting outcomes of NSM for cancer at 13-year median follow-up was from the Karolinska Institutet in Sweden and reported by Benediktsson et al. (53). They followed 216 patients from 1988 to 1994 who underwent unilateral NSM for a variety of cancers (13.3% DCIS, 33.3% stage I, 37.9% stage II, 15.3% stage III) and showed a DFS of 51.3%, OS of 76.4%, LRR of 24.1%, and DM rate of 20.3%. The OS rates were considered acceptable compared to other Swedish trials of MRM at that time. The LRR was considered high and the follow-up of the patients who had a LRR (most had repeated local excisions and some with radiation therapy as well) showed no effect on their OS which is not the usual outcome for patients with recurrences after mastectomy. The Benediktsson study included a high percentage of patients with multifocality (73.6%), tumors >2 cm (35%), patients with positive lymph nodes (40.3%) and also used an older, less aggressive surgical technique for full breast tissue removal which may have also accounted for the higher LRR.
Cancer specific indications are not being specifically addressed in this paper but were available in many of the articles reviewed. There is no unanimity in the selection of cancer patients across many of the articles written on NSM. It can be argued however, that given the current available studies and lack of long-term cancer outcome, careful patient selection of patients undergoing NSM should be considered. These patients typically include isolated tumors <2.5 cm, >2 cm from the nipple, and without skin involvement.
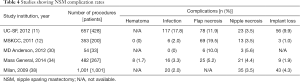
During the initial introduction of NSM to both high-risk and cancer patients, it was felt that complication rates especially for skin/nipple necrosis would be too high to justify its use. It took time to implement its use in many programs but as the initial results began showing acceptable complication rates (Table 4), the technique took hold so that its use became universally accepted though individual selection criteria vary. Nipple and skin flap necrosis rates have typically fallen to rates between 5–10% with most being treated conservatively without full nipple loss. Complication rates have also fallen with improved experience and technical improvements as was seen in the papers by Garwood et al. (13) and Colwell et al. (34) where keeping the incisions away from the nipple (encompassing <30% of the NAC) and using inframammary incisions improved their complications. Better understanding of the NAC and skin flap blood supply as shown by O’dey et al. (36) and van Deventer (35) have also improved our ability to place incisions away from the major blood supply, the medial internal mammary artery, and have helped us understand the ability of the nipple to survive with minimal 2–3 mm rims of residual periareolar tissue while still removing the majority of the ductal tissue.
Full table
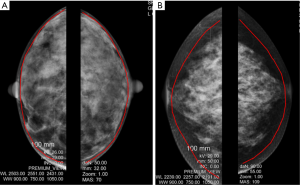
Developing the skin flaps and preserving blood supply has also been enhanced by our understanding of the vascular anatomy but it should also be noted that there is significant patient variability in skin flap thickness required to adequately remove the majority of the breast tissue while preserving the dermal blood supply. Figure 3 depicts a picture of two separate mammograms of two totally different patients where the skin flap thickness needs to be varied. A smaller lean patient will typically have a thin subdermal fat plane and thus require a thinner flap to remove all the breast tissue while maintaining the dissection in the glandular-dermal plane to keep the dermal vasculature intact. A larger patient can have a thicker subdermal fat plane, especially away from the NAC and care must be taken to not make the flap so thin that most of the subdermal fat is dissected away (lipo-dermal plane) which will remove more dermal vasculature and thus increase flap necrosis rates and complications.
The use of sharp (knife) vs. electrocautery dissection in raising the anterior skin flap is operator dependent. Sharp dissection often leads to increase blood loss and tumescence is often considered. Though there are no specific papers evaluating the risk of complications with NSM alone, tumescence has been used in NSM with acceptable outcomes (44). I personally like to use the peak PlasmaBladeTM (Medtronic, Palo Alto, CA, USA) and do not use tumescence while raising my skin flaps since the PlasmaBladeTM causes less thermal injury than the standard electrocautery due to a more precise area of action, especially when using the cutting function. I do not like the temporary vasoconstriction which occurs with the use of epinephrine with the use of tumescence. In my practice, we also use the SPY EliteTM (Novadaq, Ontario, Canada) intraoperative skin perfusion testing of the flaps after our mastectomies and after our reconstructions to evaluate our skin flap perfusion. This has helped us to better evaluate our surgical techniques in real time and gives us immediate feedback as to the vascular integrity of our flaps. Though there are few publications on its ability to predict flap necrosis in NSM (55), we find it a good qualitative perfusion test that has been able to help predict skin loss in virtually all our cases where it has occurred. The use of tumescence and associated vasoconstriction significantly affects the intraoperative perfusion testing, making the test difficult to interpret.
In the studies reviewed, patient selection factors had a significant effect on both cosmetic outcomes and complication rates. Most of the studies of NSM were highly selective in their patient populations and current smokers as well as high BMI, large or ptotic breast, or patients with prior breast irradiation were excluded from NSMs (12,24,30,56). All of these factors seem to contribute to increased complications in the various studies reviewed which analyzed these patient factors (13,32,34,47) and should be carefully considered when selecting patients as candidates for NSM.
Overall cosmesis and patient satisfaction have been shown to be good to excellent in the NSM studies reviewed (10,28,31-33,54) and there are definite improvements seen in aesthetic outcomes in the studies comparing SSM with NSM (18,33). Complication rates have been shown to negatively affect satisfaction scores to a greater degree in prophylactic mastectomy patients as compared to the patients undergoing mastectomies for cancer (57). Dissatisfaction with nipple sensation and arousal scores associated with NSMs are common (32) though there have been no technical methods shown to improve these results. The only technical factors considered in the reviewed studies that helped improve nipple necrosis complications and patient cosmesis and satisfaction were incision placement away from the nipple which deceased nipple necrosis rates (33) and non-radial incisions which decreased nipple lateralization (28,32).
There have been several methods used to try to perform NSMs on larger or ptotic breasted women. We have preferred a staged reduction mastopexy procedure as described by Spear et al. (50), especially for prophylactic patients whose surgeries can be delayed. Full-thickness nipple grafting has also been used and is well described but is associated with increased nipple losses. Dietz et al. (58) has also described a unique technique of nipple preservation with a reduction procedure performed during the surgery and preserving the dermal vessels to the NAC by deepithelializing the surrounding skin of the nipple to perform the reduction yet preserve the NAC vasculature without a full-thickness graft.
The use of an endoscopic technique in performing NSMs for cancer patients has also been described by Sakamoto et al. (59,60) from Japan who uses a combination of an axillary and periareolar incision to perform the dissection off of the pectoralis fascia and the anterior skin flaps, respectively. The axillary incision is first used for the SLN biopsy. They had good results in their initial paper (59) including 87 patients from 2002 to 2005 but noted significantly higher rates of nipple necrosis with nipple coring (41%) vs. non-coring (18%) of the nipple. The follow-up paper from 2016 (60) included 404 patients and 421 breasts with a very acceptable LRR of 2.6% after a median follow-up of 61 months. Age <40 years, stage III cancer, and inadequate surgical margins were significant variables associated with LRR.
In regards to reconstructive techniques, current studies would suggest that autologous reconstruction is associated with increased complication rates when performed with NSMs (13,26,47). Despite these initial concerns, autologous reconstruction has its benefits and should not be abandoned as an option in well selected patients. In our particular practice, direct to implant reconstruction has given excellent immediate cosmetic results in the majority of patients undergoing NSM with acceptable complication rates and can still be an option in larger or ptotic patients in combination with prior reduction mastopexy (50). It also has the added benefit of avoiding an unnecessary second procedure for the patient. We routinely use acellular dermal matrix (Alloderm, Allergan, Dublin, Ireland) for our direct to implant reconstructions and have not seen any specific increase in complication rates (61) and have even seen benefits in reduced capsular contracture rates, even in irradiated patients (62).
Conclusions
The technical aspects of NSM surgery vary from practice to practice in the literature and each surgeon has their own biases. The purpose of this review was by no means to endorse any one specific technique but to give an overview of the complexities of the surgery which exist and to offer certain principles and methods to help lower complications rates to get the best results from a standpoint of patient satisfaction and cancer outcome. The best take away information that can be gleamed from my personal experience and this literature review is that nipple viability can best be preserved by not encompassing more than 30% of the nipple in any incision and keeping a 2–3 mm radius of tissue around the nipple bundle when performing the dissection underneath the nipple. The incision should be kept away from the nipple, preferably in the lateral or inferior aspects of the breast, to preserve the blood supply to the skin flaps and to offer the best cosmetic satisfaction results. Skin flaps need to be handled with care (blunt fiberoptic lighted retractors) and the anterior flap needs to be dissected in the glandular dermal plane, leaving as much of the subdermal fat intact (again to prevent vascular damage). This plane varies in thickness from patient to patient. The use of sharp dissection or electrocautery or the peak PlasmaBladeTM (Medtronic, Palo Alto, CA, USA) is at the discretion of the individual surgeon. Patient variables such as BMI, breast size, the need for postoperative radiation, and smoking must also be considered with changes in the technique such as staged reductions, the use of expanders, or even the decision to abandon a NSM and perform a SSM with removal of the nipple in select cases. Coordinated planning of the surgery with an experienced cancer and reconstructive team is of utmost importance to obtain the best patient outcomes. Continual monitoring of complication rates as well as cancer specific outcomes will also ensure the best quality of care for your patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Ashikari is a consultant for LifeCell Corp. The other authors have no conflicts of interest to declare.
References
- Laronga C, Kemp B, Johnston D, et al. The incidence of occult nipple-areola complex involvement in breast cancer patients receiving a skin-sparing mastectomy. Ann Surg Oncol 1999;6:609-13. [Crossref] [PubMed]
- Lagios MD, Gates EA, Westdahl PR, et al. A guide to the frequency of nipple involvement in breast cancer. A study of 149 consecutive mastectomies using a serial subgross and correlated radiographic technique. Am J Surg 1979;138:135-42. [Crossref] [PubMed]
- Simmons RM, Brennan M, Christos P, et al. Analysis of nipple/areolar involvement with mastectomy: can the areola be preserved? Ann Surg Oncol 2002;9:165-8. [Crossref] [PubMed]
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999;340:77-84. [Crossref] [PubMed]
- Meijers-Heijboer H, van Geel B, van Putten WL, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 2001;345:159-64. [Crossref] [PubMed]
- Schrag D, Kuntz KM, Garber JE, et al. Decision analysis--effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med 1997;336:1465-71. [Crossref] [PubMed]
- Grann VR, Panageas KS, Whang W, et al. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J Clin Oncol 1998;16:979-85. [Crossref] [PubMed]
- Hartmann LC, Sellers TA, Schaid DJ, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst 2001;93:1633-7. [Crossref] [PubMed]
- Contant CM, Menke-Pluijmers MB, Seynaeve C, et al. Clinical experience of prophylactic mastectomy followed by immediate breast reconstruction in women at hereditary risk of breast cancer (HB(O)C) or a proven BRCA1 and BRCA2 germ-line mutation. Eur J Surg Oncol 2002;28:627-32. [Crossref] [PubMed]
- Sacchini V, Pinotti JA, Barros AC, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg 2006;203:704-14. [Crossref] [PubMed]
- Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol 2012;19:3402-9. [Crossref] [PubMed]
- de Alcantara Filho P, Capko D, Barry JM, et al. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol 2011;18:3117-22. [Crossref] [PubMed]
- Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg 2009;249:26-32. [Crossref] [PubMed]
- Coopey SB, Tang R, Lei L, et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol 2013;20:3218-22. [Crossref] [PubMed]
- Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25:5203-9. [Crossref] [PubMed]
- King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol 2011;29:2158-64. [Crossref] [PubMed]
- Yao K, Stewart AK, Winchester DJ, et al. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998-2007. Ann Surg Oncol 2010;17:2554-62. [Crossref] [PubMed]
- Gerber B, Krause A, Reimer T, et al. Skin-sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction is an oncologically safe procedure. Ann Surg 2003;238:120-7. [Crossref] [PubMed]
- De La Cruz L, Moody AM, Tappy EE, et al. Overall Survival, Disease-Free Survival, Local Recurrence, and Nipple-Areolar Recurrence in the Setting of Nipple-Sparing Mastectomy: A Meta-Analysis and Systematic Review. Ann Surg Oncol 2015;22:3241-9. [Crossref] [PubMed]
- Boughey JC, Khakpour N, Meric-Bernstam F, et al. Selective use of sentinel lymph node surgery during prophylactic mastectomy. Cancer 2006;107:1440-7. [Crossref] [PubMed]
- Black D, Specht M, Lee JM, et al. Detecting occult malignancy in prophylactic mastectomy: preoperative MRI versus sentinel lymph node biopsy. Ann Surg Oncol 2007;14:2477-84. [Crossref] [PubMed]
- Soran A, Falk J, Bonaventura M, et al. Is routine sentinel lymph node biopsy indicated in women undergoing contralateral prophylactic mastectomy? Magee-Womens Hospital experience. Ann Surg Oncol 2007;14:646-51. [Crossref] [PubMed]
- Colwell AS, Gadd M, Smith BL, et al. An inferolateral approach to nipple-sparing mastectomy: optimizing mastectomy and reconstruction. Ann Plast Surg 2010;65:140-3. [Crossref] [PubMed]
- Stolier AJ, Levine EA. Reducing the risk of nipple necrosis: technical observations in 340 nipple-sparing mastectomies. Breast J 2013;19:173-9. [Crossref] [PubMed]
- Salibian AH, Harness JK, Mowlds DS. Inframammary approach to nipple-areola-sparing mastectomy. Plast Reconstr Surg 2013;132:700e-8e. [Crossref] [PubMed]
- Endara M, Chen D, Verma K, et al. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg 2013;132:1043-54. [Crossref] [PubMed]
- Crowe JP, Patrick RJ, Yetman RJ, et al. Nipple-sparing mastectomy update: one hundred forty-nine procedures and clinical outcomes. Arch Surg 2008;143:1106-10; discussion 1110. [Crossref] [PubMed]
- Salgarello M, Visconti G, Barone-Adesi L. Nipple-sparing mastectomy with immediate implant reconstruction: cosmetic outcomes and technical refinements. Plast Reconstr Surg 2010;126:1460-71. [Crossref] [PubMed]
- Wijayanayagam A, Kumar AS, Foster RD, et al. Optimizing the total skin-sparing mastectomy. Arch Surg 2008;143:38-45; discussion 45. [Crossref] [PubMed]
- Wagner JL, Fearmonti R, Hunt KK, et al. Prospective evaluation of the nipple-areola complex sparing mastectomy for risk reduction and for early-stage breast cancer. Ann Surg Oncol 2012;19:1137-44. [Crossref] [PubMed]
- Yueh JH, Houlihan MJ, Slavin SA, et al. Nipple-sparing mastectomy: evaluation of patient satisfaction, aesthetic results, and sensation. Ann Plast Surg 2009;62:586-90. [Crossref] [PubMed]
- Djohan R, Gage E, Gatherwright J, et al. Patient satisfaction following nipple-sparing mastectomy and immediate breast reconstruction: an 8-year outcome study. Plast Reconstr Surg 2010;125:818-29. [Crossref] [PubMed]
- Moyer HR, Ghazi B, Daniel JR, et al. Nipple-sparing mastectomy: technical aspects and aesthetic outcomes. Ann Plast Surg 2012;68:446-50. [Crossref] [PubMed]
- Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg 2014;133:496-506. [Crossref] [PubMed]
- van Deventer PV. The blood supply to the nipple-areola complex of the human mammary gland. Aesthetic Plast Surg 2004;28:393-8. [Crossref] [PubMed]
- O'Dey Dm, Prescher A, Pallua N. Vascular reliability of nipple-areola complex-bearing pedicles: an anatomical microdissection study. Plast Reconstr Surg 2007;119:1167-77. [Crossref] [PubMed]
- Spear SL, Willey SC, Feldman ED, et al. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast Reconstr Surg 2011;128:1005-14. [Crossref] [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: one thousand and one cases of a five years experience at the European institute of oncology of Milan (EIO). Breast Cancer Res Treat 2009;117:333-8. [Crossref] [PubMed]
- Rusby JE, Brachtel EF, Taghian A, et al. George Peters Award. Microscopic anatomy within the nipple: implications for nipple-sparing mastectomy. Am J Surg 2007;194:433-7. [Crossref] [PubMed]
- Palmieri B, Baitchev G, Grappolini S, et al. Delayed nipple-sparing modified subcutaneous mastectomy: rationale and technique. Breast J 2005;11:173-8. [Crossref] [PubMed]
- Jensen JA, Lin JH, Kapoor N, et al. Surgical delay of the nipple-areolar complex: a powerful technique to maximize nipple viability following nipple-sparing mastectomy. Ann Surg Oncol 2012;19:3171-6. [Crossref] [PubMed]
- Mitchell SD, Beitsch PD, Willey SC, et al. Sub-areolar tissue specimen assessment in nipple sparing mastectomies: A preliminary analysis of the American Society of Breast Surgeons Nipple Sparing Mastectomy Registry. Society of Surgical Oncology’s 66th Annual Cancer Symposium, March 6-9, 2013.
- Staradub VL, Morrow M. Modified radical mastectomy with knife technique. Arch Surg 2002;137:105-10. [Crossref] [PubMed]
- Khavanin N, Fine NA, Bethke KP, et al. Tumescent technique does not increase the risk of complication following mastectomy with immediate reconstruction. Ann Surg Oncol 2014;21:384-8. [Crossref] [PubMed]
- Abbott AM, Miller BT, Tuttle TM. Outcomes after tumescence technique versus electrocautery mastectomy. Ann Surg Oncol 2012;19:2607-11. [Crossref] [PubMed]
- Khavanin N, Jordan S, Lovecchio F, et al. Synergistic interactions with a high intraoperative expander fill volume increase the risk for mastectomy flap necrosis. J Breast Cancer 2013;16:426-31. [Crossref] [PubMed]
- Gould DJ, Hunt KK, Liu J, et al. Impact of surgical techniques, biomaterials, and patient variables on rate of nipple necrosis after nipple-sparing mastectomy. Plast Reconstr Surg 2013;132:330e-8e. [Crossref] [PubMed]
- Crowe JP Jr, Kim JA, Yetman R, et al. Nipple-sparing mastectomy: technique and results of 54 procedures. Arch Surg 2004;139:148-50. [Crossref] [PubMed]
- Ashikari RH, Ashikari AY, Kelemen PR, et al. Subcutaneous mastectomy and immediate reconstruction for prevention of breast cancer for high-risk patients. Breast Cancer 2008;15:185-91. [Crossref] [PubMed]
- Spear SL, Rottman SJ, Seiboth LA, et al. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg 2012;129:572-81. [Crossref] [PubMed]
- Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull 1962;30:676-82. [Crossref] [PubMed]
- Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010;304:967-75. [Crossref] [PubMed]
- Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol 2008;34:143-8. [Crossref] [PubMed]
- Gerber B, Krause A, Dieterich M, et al. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg 2009;249:461-8. [Crossref] [PubMed]
- Wapnir I, Dua M, Kieryn A, et al. Intraoperative imaging of nipple perfusion patterns and ischemic complications in nipple-sparing mastectomies. Ann Surg Oncol 2014;21:100-6. [Crossref] [PubMed]
- Garcia-Etienne CA, Cody Iii HS 3rd, Disa JJ, et al. Nipple-sparing mastectomy: initial experience at the Memorial Sloan-Kettering Cancer Center and a comprehensive review of literature. Breast J 2009;15:440-9. [Crossref] [PubMed]
- Colakoglu S, Khansa I, Curtis MS, et al. Impact of complications on patient satisfaction in breast reconstruction. Plast Reconstr Surg 2011;127:1428-36. [Crossref] [PubMed]
- Dietz J, Fedele G. Skin Reduction Nipple-Sparing Mastectomy. Ann Surg Oncol 2015;22:3404. [Crossref] [PubMed]
- Sakamoto N, Fukuma E, Higa K, et al. Early results of an endoscopic nipple-sparing mastectomy for breast cancer. Ann Surg Oncol 2009;16:3406-13. [Crossref] [PubMed]
- Sakamoto N, Fukuma E, Teraoka K, et al. Local recurrence following treatment for breast cancer with an endoscopic nipple-sparing mastectomy. Breast Cancer 2016;23:552-60. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Berry C, et al. Acellular Dermal Matrix-Assisted Direct-to-Implant Breast Reconstruction and Capsular Contracture: A 13-Year Experience. Plast Reconstr Surg 2016;138:329-37. [Crossref] [PubMed]


