The evolution of mastectomy surgical technique: from mutilation to medicine
Since the first description of breast surgery over 3,000 years ago, anatomical and surgical techniques in the field of breast surgery have undergone an enormous amount of innovation and upheaval. From early descriptions discouraging any intervention for breast tumors due to extensive chest wall disfigurement, breast surgical oncology offers insight into the history of medicine and moreover the close relationship surgical and medical treatment modalities must maintain in order to treat patients most effectively.
The earliest reports of breast cancer are found in the Edwin Smith Egyptian papyrus. These documents, likely originating between 3,000 BCE and 1,500 BCE, were named for an archaeologist collector who bought and translated the documents in 1862. While other papyri from the time period describe managing diseases with magic, the Edwin Smith papyrus is the oldest known surgical document. This piece of history recorded 48 cases of traumatic injuries and tumors. Breast tumors were generally treated with simple cautery, however case 45, titled, “Instructions Concerning Tumours of His Breast” advocated for no treatment of the disease, as there was believed to be no cure (1). The assumed morbidity of the disease will be retracted and reinstated in cycles throughout the history of breast surgical oncology.
In the Classical Greek period, Hippocrates (b. 460 BCE), one of the forefathers of medicine, put forth the “humoral theory of medicine”. This theory posited that the body was made up of four humors: blood, phlegm, yellow bile, and black bile. He believed that health was maintained by balanced levels of these humors, while unbalanced humors manifested in disease states. In his writings, he described a breast tumor associated with bloody nipple discharge and believed this was due to an excess of black bile within the body (2).
Hippocratic thought dominated medical thinking for three centuries into the Greco-Roman period (150 BCE to 500 AD). The Roman Aurelius Celsus (b. 25 BCE) wrote De Medicina in 30 AD, in which he described a fixed irregular swelling of the female breast with tortuous veins and ulceration (3). He recognized that treating anything past early cancer development had a high rate of recurrence, and recommended against surgical procedures for advanced breast cancer. Leonides, a surgeon who broke with Hippocratic teaching, was perhaps the first breast oncologic surgeon. He advocated for alternating incision and cautery with complete removal of the tumor in the first century (4).
The Greek physician and philosopher Galen, born in modern day Turkey in 129 AD, is one of the most influential and important ancient medical scholars. He performed numerous animal dissections and recognized the similarity to humans. Through his travels to Crete, Corinth, Cyprus, and Alexandria, Galen was able to expand his knowledge of human anatomy and surgical principles. He distinguished venous from arterial blood, realized the brain controlled the body through nerves, and even separated sensory and motor neurons in his dissections. He was an accomplished surgeon, tending to the gladiators in the Roman Empire. Galen ascribed to the Hippocratic humoral theory, and paid particular attention to black bile’s supposed carcinogenic effects. Similar to Aurelius Celsus, he described breast cancer as a swelling with distended veins, which he compared to a crab. Indeed, karkinos, the etymological basis of carcinoma, is Greek for “crab”. Galen suggested en bloc extirpation of the swelling and its crablike projections. Although he warned of hemorrhagic potential during the procedure, Galen described the therapeutic effects of releasing the black bile from the body (5).
Between the fall of the Roman Empire and the beginning of the Renaissance, Galen’s humoral theory permeated most medical thought. During this period, the management of breast tumors remained relatively unchanged. Rhazes of Persia, one of the great Arabic doctors, recommended excision and cautery only if the tumor could be removed entirely—otherwise avoiding surgery, while the writings of Paul of Aegina (b. 625) and Lafranc of Milan (b. 1250) recommended excision and cautery. Medical thought was stifled somewhat throughout Europe as monks were forbidden from studying medicine and surgery after the Council of Rheims [1131] and the Council of Tours [1163], respectively.
The Renaissance saw the ballooning of innovation across engineering, navigation, and medicine. The printing press allowed rapid dissemination of information, with new scientific discoveries emanating from universities and centers of learning across Europe. It was nearly 1,500 years from Galen’s animal anatomical studies before a single human anatomy textbook was published; the first comprehensive human anatomy textbook, De Humani Corporis Fabrica Libri Septem, was published by Andreas Vesalius in 1543. With this new knowledge came little development in breast oncology, as Vesalius maintained the status quo and recommended excision. He did argue against cautery for bleeding vessels—instead opting for ligatures to maintain hemostasis.
Ambrose Paré (b. 1510) of Paris became a well-known surgeon from his experience treating injured soldiers. A firm practitioner of the scientific method, Paré advocated for a more graded approach to breast surgery. Superficial cancers could be excised while more advanced cancers were compressed with lead plates in an effort to reduce the blood supply to the mass. Perhaps most importantly, he was the first to notice swelling of the axillary “glands” in advanced breast cancer. The Spanish physician Michael Servetus (b. 1509) also studied in Paris, and was the first to advocate for an axillary node dissection as well as removal of a portion of the pectoralis muscles when removing breast tumors. Paré and Servetus’ ideas preceded Dr. William S. Halsted’s landmark paper by over 300 years.
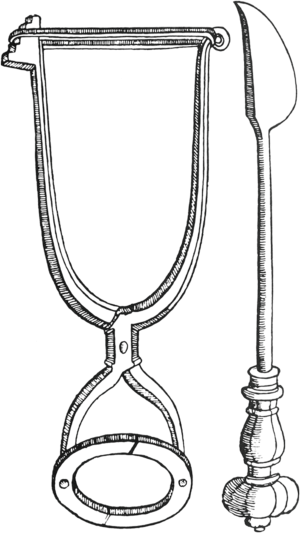
In the 1500s, several German surgeons made major contributions in the field of surgery. William Fabry (b. 1560), the father of German surgery, devised an instrument (Figure 1) to compress and fix the base of a breast during mastectomy (7). This allowed for rapid excision of the breast, as was necessary prior to development of anesthetics. Johann Schultes (b. 1595) described using heavy ligatures to obtain anterior traction, which also allowed swift dissection of breast tissue. Regardless of these detailed techniques and case reports, few mastectomies were actually being performed during this time due to the paucity of skilled surgeons and the excessive disfigurement, morbidity, and mortality associated with the procedure itself.
In the 18th century discoveries in medicine and surgery were slow to develop. However, major contributions in lymph node mapping during this period are attributed to Pieter Camper (b. 1722) and Paolo Mascagni (b. 1752), who described the internal mammary and pectoral lymph nodes, respectively. Henri Le Dran (b. 1685) wrote that cancer begins as a local disease but then spreads via lymph, which conveyed a grave prognosis (8). Le Dran’s colleague, Jean Petit (b. 1674), recommended breast, pectoral muscle, and axillary lymph node removal in the management of breast cancer (9). However, without reconstructive capabilities, the chest wall was often left disfigured with a large gaping wound. These procedures were also being performed without aseptic technique, thus the mortality was still prohibitive. Mastectomies were prevalent early in the 1700s, but by late in the century, attempts at mastectomy became extremely rare.
The 19th century dramatically changed the field of surgery. In 1804, Japanese surgeon Seishu Hanaoka (Figure 2) performed the world’s first procedure under general anesthesia—a mastectomy. He had worked tirelessly to concoct an anesthetic potion (named Tsusensan) after learning of ancient Chinese physician Hua Tuo’s success (11). In the Western World, William Morton demonstrated the use of ether for anesthesia in 1846 and Joseph Lister described antiseptic technique in 1867. The 19th century also saw an increasing prevalence of statistical analyses of surgical results rather than publications describing techniques without longitudinal results.

However, despite these innovations, the confusion over breast oncologic surgery continued. Sir James Paget (b. 1814), who described Paget’s disease of the breast, published statistics showing a 10% mortality rate of 235 patients undergoing mastectomy, with the remainder having cancer recurrence within 8 years of follow-up (12). Charles Moore (b. 1821) published the widely-accepted paper, “On the Influence of Inadequate Operation on the Theory of Cancer” in 1867, which stated that wide resection of cancer is necessary because more advanced stages were caused by dispersion from a primary tumor (13). In 1844, Jean-Jacques-Joseph Leroy d’Etiolles analyzed 1,192 patients and found mastectomy to be more harmful than helpful. German surgeons Volkmann, Billroth, Kuster, and Heidenhain advocated slightly different approaches to mastectomy, with some advocating removal of the entire breast regardless of stage, others recommending local excision for early cancers, some advocating removing all axillary contents including fat, and some advocating muscle excision. Overall, there was little standard of care despite the wide variety of options and opinions available.
In the United States, breast surgery during the 19th century was also characterized by individual surgeons advocating their specific techniques. Joseph Pancoast described an en bloc resection with vivid illustrations in 1844 (Figure 3). Samuel D. Gross described a conservative resection with axillary dissection only when the lymph nodes were obviously involved (15). His son, Samuel W. Gross who had the benefit of operating under aseptic techniques described by Lister in 1867 advocated for the “dinner plate operation” with radical dissection of the breast, skin, paramammary fat, pectoral fascia, and axillary contents (16). On the contrary, D. Hayes Agnew shared the pessimism prevalent among some surgeons of the time, believing that few cancers could truly be cured by resection alone (17).
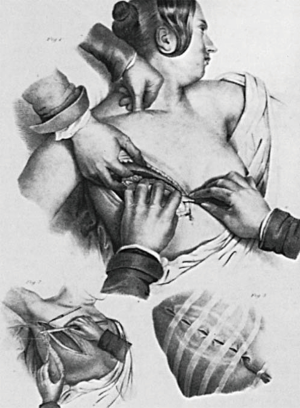
In 1894 William S. Halsted (Figure 4) published the landmark paper, The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June 1889, to January 1894. He recommended en bloc resection of all suspected tissues including the pectoralis major muscle, which became known as the “Halsted radical mastectomy” (18). Halsted’s work continued to evolve over his career, and his later ideas advocated for even further dissection of the fascia of the rectus abdominis, the serratus anterior, the subscapularis, the latissiumus dorsi, and the teres major muscle (19,20). Obviously, he was not the first to describe wide extirpation. However, his publications were scientific in nature: very clear in procedure with descriptions of every patient’s procedure (Figure 5) and each patient’s respective outcome. Halsted touted a local recurrence rate of just seven percent, unmatched by his peers. Furthermore, he worked tirelessly to spread his procedure among breast surgeons of the time, including the trainees in his residency program, the first surgical residency in the United States. Just ten days after Halsted published his landmark paper, Willie Meyer of New York published a paper describing almost the identical procedure, but he preferred pectoralis minor resection in addition to the pectoralis major (22). However it was Halsted’s technique that prevailed for 70 years.

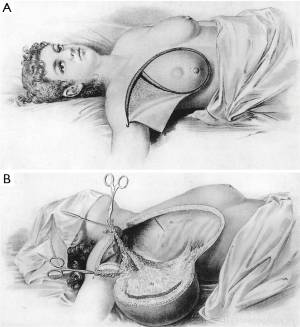
After the widespread acceptance of Halsted’s radical mastectomy, some notable surgeons such as Jerome Urban and Owen Wangensteen advocated even further resections that included the internal mammary lymph nodes and supraclavicular lymph nodes—a “supraradical mastectomy” (23,24). However, results from more extensive surgeries showed no increased survival, and as the 20th century progressed, it became clear to most surgeons that medical advancements rather than further surgical advancements had the most promise in curing cancer.
The discovery of X-rays by William Roentgen in 1895 changed the landscape of medicine and led to the shift of breast cancer treatment during the 20th century from purely surgical to the multiple modalities employed today. Initial work with X-rays was fraught with damage to skin and superficial tissue from radiation toxicity (25). Nevertheless, by 1902 Russian physicist S. Goldberg successfully used radiation for cancer treatment, and the following year the first department for radiotherapy was established at the Cancer Hospital in London. By the early 20th century radiotherapy began to gain traction as a treatment option for breast cancer patients deemed inoperable, as well as those with recurrent disease despite radical mastectomy. However, implications of long-term radiation exposure with local toxicity and de novo cancer development precluded initial widespread use of radiation therapy for breast cancer treatments, until continued advancements in radiotherapy delivery over the following decades employed higher voltage treatments with improved focused targeting to tissues with tumor burden.
In 1937, Geoffrey Keynes of London demonstrated that radiation therapy and mastectomy had equivocal outcomes in the management of breast cancer, but the therapy did not gain popularity and radical mastectomy continued as the standard of care into the 1940s. However, in 1948 the modified radical mastectomy, which spared the pectoralis muscles, was introduced by Patey and Dyson from Middlesex Hospital in London (26). In the same year, simple mastectomy combined with radiotherapy was introduced by McWhirter in Edinburgh. These two methods were studied subsequently and showed that patients treated with simple, radical, or modified radical mastectomies with or without radiotherapy had strikingly similar outcomes. This led to the birth of breast conservation therapy for breast cancer.
By the 1970’s, advances in cancer biology and disease understanding corroborated the concept of breast conservation surgery. Furthermore the increasingly widespread use of mammography allowed for earlier disease detection and greater opportunities to study evolving treatment modalities. The Milan trials demonstrated no difference in 5-year survival rates between quadrantectomy plus radiotherapy plus axillary dissection versus radical mastectomy (27), and the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-04 trial by Fisher et al. successively proved equivalent 5-year survival when comparing lumpectomy versus removal of all breast tissue, pectoral fascia, and axillary contents (28). These studies heralded the paradigm shift away from the mutilation previously believed to be necessary for disease control. Indeed, lumpectomy and radiotherapy has become the standard management of stage I/II breast cancer in the present day, with the total mastectomy withheld for patients with more advanced or diffuse disease, inflammatory breast cancer, and recurrent disease following initial conservation surgery (29).
The current landscape of breast cancer treatment encompasses a wide assortment of imaging modalities, chemotherapy, targeted hormonal therapy, improvements in radiation targeting, and targeted immunotherapy. What was once thought to be impossible to cure just over 150 years ago has treatment options for patients at each stage of disease. Although breast cancer continues to rank highest among frequently diagnosed cancers in US women with an incidence of approximately 246,660 cases in 2016, mortality rates continue to decrease yearly with an overall regression of 36% from 1989 to 2012 (30).
To achieve this decline, current care typically consists of a multi-disciplinary approach combining the expertise of oncologic surgeons, plastic surgeons, radiologists, medical oncologists, radiation oncologists, pathologists, psychologists, and nurses. The improvement in survival trends has mirrored the increased focus on quality of life. While disease control remains the primary aim of breast cancer treatment, improved aesthetic outcomes via reconstruction continues to positively impact patient’s lives after tumor extirpation (31).
From this ideal, the concept of oncoplastic breast surgery has emerged. Not surprisingly, new surgical procedures have arisen which, in conjunction with previously described techniques and a myriad of adjuvant therapies, work to individually tailor treatment to each patient’s needs.
Although Freeman first described the subcutaneous or skin preserving mastectomy for benign breast disease in 1962 (32), it wasn’t until 1991 that Toth and Lappert coined the skin-sparing mastectomy for breast cancer treatment (33). The operation consists of removal of all breast tissue and nipple areola complex (NAC) in an elliptical incision pattern, with additional removal of skin overlying the tumor if superficially located. The remaining breast skin is preserved in order to facilitate breast reconstruction. If a sentinel lymph node biopsy or axillary dissection is indicated, these can be performed through the same incision following removal of the breast mound. Through this approach, some residual breast tissue can remain on skin flaps, especially if left with >5 mm thickness (34). However, recent literature supports the claims that it is near technically impossible to remove all breast tissue with any mastectomy technique (35,36), and increasing evidence reports that loco-regional recurrence is related more to tumor biology than surgical technique (37,38). A meta-analysis published in 2010 comparing skin-sparing mastectomy to conventional mastectomy showed no difference in local recurrence between the two procedures (39), and a successive large retrospective review from MD Anderson of 1,810 patients demonstrated no significant difference in local, regional, or systemic recurrence rates between the two procedures at a median follow-up of 53 months (40). Thus, skin-sparing mastectomy is oncologically safe and allows for improved aesthetic outcomes with proper reconstructive planning.
The NAC is an essential portion of naturally appearing breasts, and loss of the NAC during total or skin-sparing mastectomy is associated with adverse psychological consequences related to worsening body image and feelings of mutilation (41). Multiple techniques to reconstruct the NAC exist; however these still confer possible loss of nipple projection and difficulties creating natural appearing areola pigmentation and surface texture despite excellent tattoo artistry.
The nipple-sparing mastectomy was therefore developed to remove all breast glandular tissue with total preservation of skin and NAC. The benefit of this procedure lies in its superior cosmetic outcomes, making it an ideal procedure in a growing population of patients attracted to the potential of a virtually unchanged breast appearance, except a small scar, after reconstruction. Breast cancer patients and those desiring prophylactic mastectomy due to high-risk genetic mutations such as BRCA-1 and BRCA-2 have increasingly popularized the nipple-sparing mastectomy in recent years. However, in order to ensure oncologic safety in those receiving treatment for existing disease determination of true surgical candidates is of utmost importance. Contraindications to nipple sparing mastectomy include carcinoma invading the skin and/or NAC (defined as cancer <2 cm from NAC), pathologic discharge from the nipple, Paget’s disease of the breast, previous radiotherapy, active smoking, obesity (BMI >30 kg/m2), and large breasts with grade-3 ptosis due to increased risk of asymmetry or nipple necrosis through poor vascular supply (42).
The procedure is typically performed either by incising through the skin-areola interface, making an S incision lateral to the NAC, or through the infra-mammary fold. Breast glandular tissue is dissected away from subcutaneous fat, attempting to preserve the dermal and sub-dermal vascular arcades. The NAC is elevated in a superficial plane via sharp dissection, avoiding thermal damage from electrocautery. The nipple is then everted and the retro-areolar tissue is transected and sent for frozen pathology.
If intra-operative pathology reports are positive for malignancy, the nipple areolar complex is excised. Following removal of remaining breast tissue, immediate or delayed reconstruction is possible depending on confidence in mastectomy flap viability and NAC vascularity. One report of oncologic safety of nipple-sparing mastectomy by Gerber et al. demonstrated similar rates of loco-regional recurrence when compared to modified radical mastectomy or skin sparing mastectomy after a 10-year follow-up period (43).
Carcinoma of the breast has been prevalent in human society since at least the ancient Egyptian times, and treatment of the disease is a continually evolving field of medicine that has mirrored the great advancements throughout recorded history. Surgical management of breast cancer has transformed from a radicalized procedure that left patients with severe morbidity to an elegant operation that delicately balances oncologic safety with reconstructive principles. Current trends in breast cancer involve a multi-modality approach founded in evidence-based practices, although surgical extirpation of breast tumors will likely remain at the forefront of treatment options for many years to come.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Breasted JH. The Edwin Smith surgical papyrus. Classics of Medicine Library. Chicago: University of Chicago Press, 1930.
- Elston CW, Ellis IO. The Breast. Edinburgh: Churchill Livingstone, 1998.
- Donaldson IM. Celsus: De medicina, Florence 1478. Part 1. J R Coll Physicians Edinb 2014;44:252-4. [Crossref] [PubMed]
- Iavazzo CR, Trompoukis C, Siempos II, et al. The breast: from Ancient Greek myths to Hippocrates and Galen. Reprod Biomed Online 2009;19 Suppl 2:51-4. [Crossref] [PubMed]
- Sakorafas GH, Safioleas M. Breast cancer surgery: an historical narrative. Part I. From prehistoric times to Renaissance. Eur J Cancer Care (Engl) 2009;18:530-44. [Crossref] [PubMed]
- Robinson JO. Treatment of breast cancer through the ages. Am J Surg 1986;151:317-33. [Crossref] [PubMed]
- Fabricius H. Observationum et curationum chirurgicarum centuriae: Cent II. Lugduni: IA Huguetan, 1641.
- Le Dran F. Memoire avec une précis de plusieurs observations sur le. Mem Acad Roy Chir Paris 1757;3:1.
- Petit JL. Ouevres Complétes, Section VII. Limoges: R. Chapoulard, 1837.
- Dote K, Ikemune K, Desaki Y, et al. Two Japanese Pioneers in Anesthesiology: Seishū Hanaoka and Gendai Kamada. J Anesth Hist 2017;3:19-23. [Crossref] [PubMed]
- Izuo M. Medical history: Seishu Hanaoka and his success in breast cancer surgery under general anesthesia two hundred years ago. Breast Cancer 2004;11:319-24. [Crossref] [PubMed]
- Paget J. On the average duration of life in patients with scirrhus cancer of the breast. Lancet 1856;1:62. [Crossref]
- Moore CH. On the Influence of Inadequate Operations on the Theory of Cancer. Med Chir Trans 1867;50:245-80. [PubMed]
- Pancoast J. A Treatise on Operative Surgery. Philadelphia: Carey and Hart, 1844.
- Gross SD. System of surgery. 5th ed. Philadelphia: Henry C Lea’s Son, 1872.
- Gross SW. An analysis of two hundred and seven cases of carcinoma of the breast. Med News 1887;51:613.
- Agnew DH. The principles and practice of surgery. Philadelphia: JB Lippincott, 1883.
- Halsted WS. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg 1894;20:497-555. [Crossref] [PubMed]
- Halsted WS. A clinical and histological study of certain adenocarcinomata of the breast. Ann Surg 1898;28:557. [PubMed]
- Halsted WS. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann Surg 1907;46:1. [Crossref] [PubMed]
- Cotlar AM, Dubose JJ, Rose DM. History of surgery for breast cancer: radical to the sublime. Curr Surg 2003;60:329-37. [Crossref]
- Meyer W. An improved method of the radical operation for carcinoma of the breast. Med Rec 1894;46:746.
- Urban JA, Baker HW. Radical mastectomy in continuity with en bloc resection of the internal mammary lymph chain. Cancer 1952;5:992-1008. [Crossref] [PubMed]
- Wangensteen OH. Discussion to Taylor and Wallace: Carcinoma of the breast, fifty years’ experience at the Massachusetts General Hospital. Ann Surg 1950;132:838-43.
- Sansare K, Khanna V, Karjodkar F. Early victims of X-rays: a tribute and current perception. Dentomaxillofac Radiol 2011;40:123-5. [Crossref] [PubMed]
- Patey DH, Dyson WH. The prognosis of carcinoma of the breast in relation to the type of operation performed. Br J Cancer 1948;2:7-13. [Crossref] [PubMed]
- Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med 1981;305:6-11. [Crossref] [PubMed]
- Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 1985;312:665-73. [Crossref] [PubMed]
- Fajdic J, Djurovic D, Gotovac N, et al. Criteria and procedures for breast conserving surgery. Acta Inform Med 2013;21:16-9. [Crossref] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society 2016.
- Piper M, Peled AW, Sbitany H. Oncoplastic breast surgery: current strategies. Gland Surg 2015;4:154-63. [PubMed]
- Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull 1962;30:676-82. [Crossref] [PubMed]
- Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg 1991;87:1048-53. [Crossref] [PubMed]
- Torresan RZ, dos Santos CC, Okamura H, et al. Evaluation of residual glandular tissue after skin-sparing mastectomies. Ann Surg Oncol 2005;12:1037-44. [Crossref] [PubMed]
- Barton FE Jr, English JM, Kingsley WB, et al. Glandular excision in total glandular mastectomy and modified radical mastectomy: a comparison. Plast Reconstr Surg 1991;88:389-92. [Crossref] [PubMed]
- Carlson GW, Styblo TM, Lyles RH, et al. Local recurrence after skin-sparing mastectomy: tumor biology or surgical conservatism? Ann Surg Oncol 2003;10:108-12. [Crossref] [PubMed]
- Ho CM, Mak CK, Lau Y, et al. Skin involvement in invasive breast carcinoma: safety of skin-sparing mastectomy. Ann Surg Oncol 2003;10:102-7. [Crossref] [PubMed]
- Romics L Jr, Chew BK, Weiler-Mithoff E, et al. Ten-year follow-up of skin-sparing mastectomy followed by immediate breast reconstruction. Br J Surg 2012;99:799-806. [Crossref] [PubMed]
- Lanitis S, Tekkis PP, Sgourakis G, et al. Comparison of skin-sparing mastectomy versus non-skin-sparing mastectomy for breast cancer: a meta-analysis of observational studies. Ann Surg 2010;251:632-9. [Crossref] [PubMed]
- Yi M, Kronowitz SJ, Meric-Bernstam F, et al. Local, regional, and systemic recurrence rates in patients undergoing skin-sparing mastectomy compared with conventional mastectomy. Cancer 2011;117:916-24. [Crossref] [PubMed]
- Wellisch DK, Schain WS, Noone RB, et al. The psychological contribution of nipple addition in breast reconstruction. Plast Reconstr Surg 1987;80:699-704. [Crossref] [PubMed]
- Rossi C, Mingozzi M, Curcio A, et al. Nipple areola complex sparing mastectomy. Gland Surg 2015;4:528-40. [PubMed]
- Gerber B, Krause A, Dieterich M, et al. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg 2009;249:461-8. [Crossref] [PubMed]


