Early multicentre experience of pre-pectoral implant based immediate breast reconstruction using Braxon®
Introduction
Breast cancer remains the most common cancer in the United Kingdom (UK) with a lifetime risk of one in eight for women (1). Last two decades have seen significant changes in the surgical management of breast cancer and the offer of immediate breast reconstruction (IBR) following mastectomy is currently standard practice. Skin sparing and nipple sparing mastectomy with implant-based IBR have emerged as oncologically safe treatment options with good cosmetic results (2).
The introduction of acellular dermal matrix (ADM) in 2001 by Salzberg et al. as an adjunct in subpectoral implant based reconstructions has seen a large increase in its use for IBR following mastectomies in the UK (3,4). Although initial studies reported higher complications, data in the last three years demonstrate decreasing complication rates (5,6). There are various ADM’s currently in use; the commonest ones are Strattice®, Surgimend®, Native® and Veritas®.
Subpectoral implant placement is the generally accepted method of implant-based reconstruction. Placement of implant in the submuscular pocket along with an ADM provides complete implant cover, a larger pocket and control of the inframammary fold. The advantages seen are minimal implant visibility, reduced rippling and minimal palpability of implant edges at upper pole of the reconstructed breast. However, the morbidity associated with pectoralis major detachment, animation deformity and postoperative pain remains a matter of concern (7,8).
Prepectoral implant placement and complete coverage of implant with ADM eliminates the need to detach the muscle from underlying chest wall. As this is a new technique, there is minimal data published in literature regarding its role in IBR. Braxon® (MBP Biologics, Neustadt-Glewe, Germany, licence holder Decomed, Marcon, Venezia, Italy) is a novel ADM introduced in the UK for prepectoral implant-based IBR. It is a pre-shaped, 0.6 mm thick mesh derived from porcine dermis and is wrapped around the implant ex vivo (9). We report short-term outcomes of a multicentre study from the UK, using this novel ADM in women having an IBR.
Methods
Patients
The prospective study was conducted from December 2015 to October 2016 and included all patients from three breast units in the UK (Warwick, University Hospital of North Midlands and Royal Lancaster University) who underwent a mastectomy and an implant-based IBR using Braxon®. Other inclusion criteria included no previous radiotherapy to the ipsilateral breast, an estimated breast volume of less than 550 cc and a good subcutaneous layer of fat (>1 cm in a pinch test measured at the upper and medial aspect of the breast).
Surgical technique
All patients were given an intravenous antibiotic at induction. A skin or nipple sparing mastectomy with or without an axillary procedure was performed through a periareolar, inframammary or skin reducing incisions. After the procedure, the pectoralis major muscle was left completely intact and the subcutaneous pocket was evaluated with a sizer in place. If necessary, the inframammary fold was reinforced and the axilla was closed with 2/0 PDS suture if a sentinel node biopsy or axillary clearance was performed. The prepectoral cavity was washed with saline and a suction drain was placed in the mastectomy cavity and another drain placed in the axilla in patients who had an axillary clearance. We used a 30 cm × 40 cm sheet of Braxon® which can accommodate an implant up to a size of 550 cc. In all patients, an anatomical shaped implant was used. The Braxon® ADM comes as a prepared template, which is sutured around the implant ex vivo using 2/0 PDS suture (Figure 1). The Braxon® with the implant was placed in the pre-pectoral pocket and secured to the pectoralis major muscle at the superior, medial and lateral aspect (sometimes more than 3 tacking sutures) using interrupted 2/0 PDS sutures. The wound was then closed in layers with absorbable sutures. Antibiotics were continued till the drains were removed. Patients were discharged home the following day with a drain in situ. Drains were retained for a week to 10 days or till drain output was less than 30 mL in a 24-hour period.

Data collection
The demographic details, co-morbidities, indication for surgery, operative details, immediate and delayed complications were recorded. Specific complications recorded were infection, seroma, unplanned readmission and loss of implant. A comparison was made with complications reported in the National Mastectomy and Breast Reconstruction Audit (NMBRA) (10,11).
Statistical methods
Statistical analysis was carried out using SPSS, version 21 for Windows SPSS 21.0 software (SPSS Inc., Chicago, IL, USA). Data was calculated as mean ± (SD), median (range), and frequency (percentage). Chi-square test was used for univariate analysis between different variables and occurrence of complications. The level of significance was set at 5%, P<0.05. Univariate analysis was done for complications against the variables recorded. Multivariate analysis was done using logistic regression analysis.
Results
A total of 64 patients from three centres were included in the analysis. Fourteen underwent a bilateral mastectomy and therefore 78 IBRs using Braxon® and pre-pectoral implant were included. Demographic details, tumor characteristics and treatment related details are shown in Table 1. Mean age of the cohort was 50 years with a mean body mass index (BMI) of 25.7 kg/m2. About 20% of the patients were current or ex-smokers, 10% had received neoadjuvant chemotherapy, about 3% had diabetes and 3% had previous radiation therapy to breast. Of the patients who underwent bilateral mastectomy, four patients had bilateral invasive cancers or DCIS, eight had a contralateral risk-reducing mastectomy and two patients had bilateral risk reduction. 19% of the patients had an axillary nodal clearance (ANC). The mean implant size used was 364.75 (range, 200 to 535). The mean hospital stay was 1.48 days with post-operative antibiotics for about 11.17 days on an average with most patients receiving them for about a week.
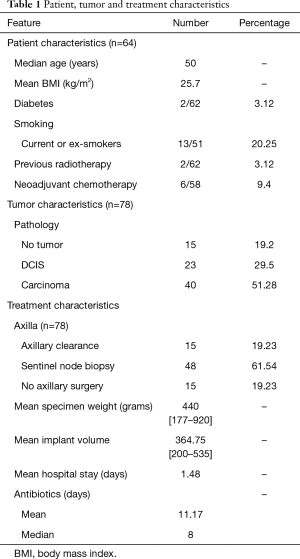
Full table
Seroma aspiration was performed in 23% of patients of whom two patients required repeat aspirations, three or more times. About 30% had a persistent erythema requiring antibiotics. Infection and hematoma rates were about 6.25% (Table 2). Twenty percent patients had a readmission within 30 days of surgery and about 22% had a re-exploration for hematoma, infection or persistent seroma. Six of 64 patients had implant removal secondary to complications; of these, two patients had bilateral implant removal. Thus of the 78 implant procedures, eight implants were removed at an explant rate of 10.2%.
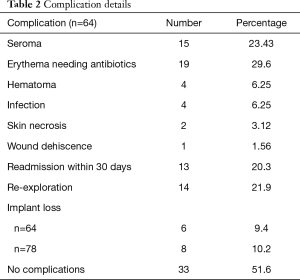
Full table
The median follow-up was 9.98 months. Of the factors (neoadjuvant chemotherapy, diabetes, smoking, type of mastectomy, implant size, BMI, ANC) thought to be contributing to post-operative complications, BMI of more than 30 kg/m2 (P=0.011) and ANC (P=0.027) were significantly associated with post-operative complications on univariate analysis. However, on multivariate analysis none of the factors were significantly associated with post-operative complications. Bilateral reconstructions were significantly associated with implant loss (P=0.018) on univariate analysis.
Discussion
The majority of implant-based IBR are performed by subpectoral placement of the implant and therefore, pain and discomfort following the elevation of pectoralis major muscle are well known postoperative complications (12). After a subpectoral IBR, most patients need opioids for at least 24 hours following surgery. By placing the implant in the prepectoral pocket, the division and stretching of pectoralis major muscle is avoided which could potentially result in a significant reduction in postoperative pain. This may enhance recovery period and reduce postoperative hospital stay. Kobraei et al. reported satisfactory patient related outcomes with shorter recovery period and earlier return to baseline activity in their small series of 13 patients with 23 prepectoral reconstructions (13). Maruccia et al. also reported an improved outcome when assessing the quality of life in women over 65 years of age who underwent prepectoral Braxon® based implant reconstruction (14). Although we have not reported specifically on the pain assessments in this study, overall the average duration of in-hospital stay was lower.
Animation deformities or breast distortion due to contraction of pectoralis major muscle is a known entity but its prevalence and significance are unclear (7,15). Loss of muscle function, breast animation and increased post-operative pain can be avoided by placing the implant in the prepectoral pocket (16). These outcomes could not be assessed due to the short follow-up of our study.
Duration of operating time has an adverse influence on wound complication and implant loss (17). In the prepectoral technique, operating time is reduced as it is a less complicated technique and the surgeon can begin to perform the ex vivo procedure of suturing the Braxon® mesh (Figure 1) around the implant after the mastectomy, while haemostasis and irrigation of the prepectoral space are being carried out. Berna et al. did not report any implant loss in their small series of 15 implant-based IBR using Braxon® and Vidya et al. in a series of 100 cases has demonstrated a very low implant loss rate of 2% (18,19). However, our complication rate of wound infection and implant loss were comparable to the NMBRA results which reported on subpectoral implant based reconstruction (Table 3) (10). Most of the other complications in our multicentre study with the prepectoral technique including symptomatic seroma, erythema and re-intervention rates were comparable to series in the literature reporting on subpectoral reconstruction with ADM. Our study therefore demonstrates that the complication rates between prepectoral and subpectoral techniques are comparable with no superiority of the prepectoral technique in this regard.
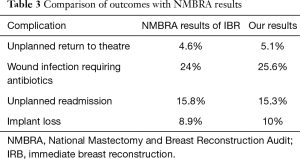
Full table
Many authors have extensively investigated capsular contracture in implant-based reconstruction. Studies have shown that the use of ADM reduces capsular contracture (20). Total wrapping of ADM has been proven to reduce capsular contracture in animal studies and in a small series of subpectoral implant-based reconstructions by Cheng et al. (21,22). Downs et al. showed promising results in their 2-year follow-up study on prepectoral implants and ADM with capsular contracture rate of 10% (17). A recent publication on a 4-year follow-up for a small series of Braxon® reconstructions showed no evidence of capsular contracture (23). The short follow- up in our study was not sufficient to assess for capsular contracture.
ADM acts as a tissue regeneration layer between the skin and implant and can lead to excess seroma formation. A recent systematic review reported a higher rate of seroma formation with ADM compared with submuscular coverage but no difference was noted in the infection rate (24). The prepectoral ADM also possibly prevents malposition and rotation of the implant and none of our patients were noted to have this complication in the short-term follow-up.
Another advantage is the potential cost effectiveness of the treatment. Although this technique requires more ADM per implant than standard approaches, it is probably still cost effective as it is a single-step reconstruction avoiding need of additional procedures and reduced hospital stay with a potential to perform these cases as a day case procedure.
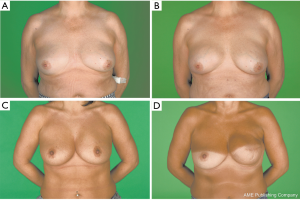
Our series represents an early experience with Braxon® for IBR with a short-term follow-up (Figure 2). It is a retrospective analysis from a prospectively maintained database and will have the disadvantages of a retrospective study (25). Variations in practice across centres and lack of a control group i.e. lack of subpectoral IBR could be significant confounders. However, the outcomes are comparable with that of historical series of subpectoral reconstructions. Cosmetic outcomes and patient-related quality of life outcomes have not been analyzed in the study.
Conclusions
Our early experience with this novel prepectoral technique using Braxon® has shown this to be an effective technique with complication rates comparable to the traditional technique of subpectoral implant with ADM. The main advantages of prepectoral implant-based IBR are quicker postoperative recovery and short post-operative hospital stay. Long term studies are required to assess rippling, post-operative animation, capsular contracture and impact of adjuvant radiotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This manuscript is a retrospective analysis of prospectively maintained data of all consecutive patients undergoing immediate prepectoral implant-based reconstruction after a mastectomy from three Breast cancer units in the United Kingdom. Oncoplastic breast surgeons routinely perform these procedures as a standard part of treatment. As no new or any experimental treatment was offered to the patients, an Ethics committee approval was not required. As this was an audit of the prospectively recorded data, it was registered and approved by the clinical audit departments of the respective NHS trusts. Informed consent was obtained from all patients in the three centres to include their treatment and management details into the hospital database for audit and governance purposes. Patients consented separately for clinical photographs to be taken (data anonymised) for hospital records, teaching, presentation in scientific meetings and publication in scientific journals.
References
- Lifetime risk of breast cancer in females in UK [Internet]. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer#heading-Zero
- Yi M, Kronowitz SJ, Meric-Bernstam F, et al. Local, regional, and systemic recurrence rates in patients undergoing skin-sparing mastectomy compared with conventional mastectomy. Cancer 2011;117:916-24. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24. [Crossref] [PubMed]
- Mennie JC, Mohanna PN, O'Donoghue JM, et al. National trends in immediate and delayed post-mastectomy reconstruction procedures in England: A seven-year population-based cohort study. Eur J Surg Oncol 2017;43:52-61. [Crossref] [PubMed]
- Agarwal JP, Mendenhall SD, Anderson LA, et al. The breast reconstruction evaluation of acellular dermal matrix as a sling trial (BREASTrial): design and methods of a prospective randomized trial. Plast Reconstr Surg 2015;135:20e-8e. [Crossref] [PubMed]
- Davila AA, Seth AK, Wang E, et al. Human Acellular Dermis versus Submuscular Tissue Expander Breast Reconstruction: A Multivariate Analysis of Short-Term Complications. Arch Plast Surg 2013;40:19-27. [Crossref] [PubMed]
- Spear SL, Schwartz J, Dayan JH, et al. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg 2009;33:44-8. [Crossref] [PubMed]
- Hammond DC, Schmitt WP, O'Connor EA. Treatment of breast animation deformity in implant-based reconstruction with pocket change to the subcutaneous position. Plast Reconstr Surg 2015;135:1540-4. [Crossref] [PubMed]
- BRAXON ADM [Internet]. Available online: http://quamedical.nl/index.php?item=braxon-adm&action=article&group_id=10000024&aid=57&lang=nl
- A national audit of provision and outcomes of mastectomy and breast reconstruction surgery for women in England. Fourth Annual Report 2011. [Cited 2017 Jun 10]. Available online: http://promesi.med.auth.gr/mathimata/clin-audi-supp-prog-mast-brea-reco-2011-rep1.pdf
- Browne J, Pereira J, Caddy CM, et al. Clinical Effectiveness Unit, The Royal College of Surgeons of England. [cited 2017 Jun 10]; Available online: https://www.rcseng.ac.uk/-/media/files/rcs/library-and-publications/non-journal-publications/national-mastectomy-and-breast-reconstruction-audit--3rd-report.pdf
- Wallace MS, Wallace AM, Lee J, et al. Pain after breast surgery: a survey of 282 women. Pain 1996;66:195-205. [Crossref] [PubMed]
- Kobraei EM, Cauley R, Gadd M, et al. Avoiding Breast Animation Deformity with Pectoralis-Sparing Subcutaneous Direct-to-Implant Breast Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e708. [Crossref] [PubMed]
- Maruccia M, Mazzocchi M, Dessy LA, et al. One-stage breast reconstruction techniques in elderly patients to preserve quality of life. Eur Rev Med Pharmacol Sci 2016;20:5058-66. [PubMed]
- Spear SL, Seruya M, Clemens MW, et al. Acellular dermal matrix for the treatment and prevention of implant-associated breast deformities. Plast Reconstr Surg 2011;127:1047-58. [Crossref] [PubMed]
- de Haan A, Toor A, Hage JJ, et al. Function of the pectoralis major muscle after combined skin-sparing mastectomy and immediate reconstruction by subpectoral implantation of a prosthesis. Ann Plast Surg 2007;59:605-10. [Crossref] [PubMed]
- Downs RK, Hedges K. An Alternative Technique for Immediate Direct-to-Implant Breast Reconstruction-A Case Series. Plast Reconstr Surg Glob Open 2016;4:e821. [Crossref] [PubMed]
- Berna G, Cawthorn SJ, Papaccio G, et al. Evaluation of a novel breast reconstruction technique using the Braxon® acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg 2017;87:493-8. [Crossref] [PubMed]
- Vidya R, Masià J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: First multicenter European report on 100 cases. Breast J 2017;23:670-6. [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Berry C, et al. Acellular Dermal Matrix-Assisted Direct-to-Implant Breast Reconstruction and Capsular Contracture: A 13-Year Experience. Plast Reconstr Surg 2016;138:329-37. [Crossref] [PubMed]
- Schmitz M, Bertram M, Kneser U, et al. Experimental total wrapping of breast implants with acellular dermal matrix: a preventive tool against capsular contracture in breast surgery? J Plast Reconstr Aesthet Surg 2013;66:1382-9. [Crossref] [PubMed]
- Cheng A, Lakhiani C, Saint-Cyr M. Treatment of capsular contracture using complete implant coverage by acellular dermal matrix: a novel technique. Plast Reconstr Surg 2013;132:519-29. [Crossref] [PubMed]
- Berna G, Cawthorn SJ. Long term follow-up on prepectoral ADM-assisted breast reconstruction: evidences after 4 years. Eur J Plast Surg 2017;40:255-8. [Crossref]
- Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg 2011;128:1162-9. [Crossref] [PubMed]
- Sedgwick P. Retrospective cohort studies: advantages and disadvantages. BMJ 2014;348:g1072. [Crossref]


