21-gene recurrence assay in patients receiving intraoperative radiotherapy: are “favorable” characteristics a surrogate for low recurrence?
Introduction
Multiple randomized studies with extensive follow-up have established breast conservation surgery (BCS) as an equivalent treatment modality to mastectomy in terms of overall survival (1-4). In these studies, adjuvant whole-breast radiotherapy was given after BCS to achieve this equivalency. The standard adjuvant treatment is daily whole breast radiotherapy for a duration of 6–7 weeks. Documented adverse effects of whole breast radiation (WBI) include skin burns and irritation, fatigue, permanent skin damage, and, less frequently, cardiac toxicity, radiation induced pneumonitis, and brachial plexopathy (5,6). Although, truncated hypofractionation protocols are shorter, a typical course of radiation will last approximately 3 weeks (7-10). This time commitment and toxicity profile has stimulated increased interest in partial breast irradiation (PBI) techniques. Intraoperative radiotherapy (IORT) is a form of PBI that allows for a single intraoperative dose of radiation to be delivered directly to the tumor bed in patients who meet the criteria and elect BCS.
IORT can be delivered by various mechanisms- including low-energy X-rays, electron beam radiation therapy (EBRT), high-dose-rate (HDR) afterloaders and other hybrid devices (11). IORT methods have been evaluated in the setting of early stage breast cancer (BC) in Europe, and, more recently, in the US with non-inferior mortality compared to WBI. The TARGeted Intraoperative radiotherapy-Alone (TARGIT-A) trial (12) was a large randomized control trial examining IORT vs. WBI (12,13). TARGIT-A data supports the use of IORT for treatment of less aggressive phenotypes based upon tumor size, hormone receptor expression and node negativity (12). The current guidelines for IORT at our institutions include unifocal invasive ductal disease, with tumor size less than 3 cm, negative margins, ER and PR positive, without lymph node involvement. These guidelines are based on TARGIT-A criteria (12).
The Oncotype DX Breast Cancer Assay (Genomic Health®) is a 21-gene assay which examines BC genes associated with proliferation and invasion. The goal of the assay is to calculate a score that serves as an estimate of the likelihood of distant recurrence. The result of the score is based on the genomic profile of the tumor. In turn, patients are stratified into high (>31), intermediate [18–30] and low (<18) risk for distant recurrence. Validation studies have shown that there is a correlation between Oncotype DX (Genomic Health®) score and risk of distant recurrence as well as response to chemotherapy (14-16). The purpose of this tool is to tailor treatment of BC to the genomic characteristics of each specific tumor. This may spare low risk patients the morbidity of undergoing adjuvant chemotherapy. At present, the selection criteria for performing a 21-gene recurrence test (RS) are early stage, ER positive, Her-2 negative, node negative invasive BC (17).
Given the published and accepted criteria for use of IORT and 21-gene RS, it is clear that there are a number of overlapping selection parameters between these prognostic and therapeutic modalities. Thus, tumors selected for IORT based on the criteria of favorable tumor characteristics, should have a low risk of recurrence. We review consecutive IORT cases performed at MGUH and MWHC in the first 2 years of its use. Our goal is to determine, using the 21-gene recurrence score, whether the early stage lesions deemed appropriate for IORT have a low risk of recurrence based on tumor genomics.
Methods
We conducted a retrospective review of patients receiving BCS and IORT between January 2013 and March 2015 at two MedStar Regional Breast Program sites: MedStar Georgetown University Hospital (GUH) and MedStar Washington Hospital Center (WHC). Institutional Review Boards at both institutions approved the study. Patients receiving IORT were entered into respective institutional registries.
Patients were identified from surgery logs and electronic medical records. We searched women age 50 and over who were preoperatively diagnosed with invasive, non-metastatic operable disease, T1 and T2 (up to 3 cm), N0, M0, confirmed by cytological or histological examination who received breast conserving surgery with IORT. These women met the criteria of eligibility to receive IORT at our institutions based on age, clinical stage, and core biopsy tumor features. At our institutions, all patients receive IORT at the time of lumpectomy. At MedStar WHC, patients with grades I–II tumors qualified for IORT. At this institution, IORT was used solely as a boost. At GUH, patients with grades I–III tumors qualified for IORT. The current guidelines for IORT at our institutions include unifocal disease, tumor size up to three centimeters, negative margins, ER and PR positive, without lymph node involvement. All patient receiving IORT as sole radiotherapy had to have at least a 1 mm margins on final pathology. At both institutions, any patient with positive or close margins underwent re-excision of margins followed by WBI, with the IORT dose acting as the boost. Patients who went on to WBI were included in the study population. Exclusion criteria included multifocal or multicentric disease, pure ductal carcinoma in situ or invasive lobular cancer. Magnetic resonance imaging (MRI) was obtained to establish unifocality at the discretion of the operating surgeon. Data collection included patient demographics (age, race), tumor characteristics (size, grade, receptor status, histopathologic characteristics, margin status results), nodal status, 21-gene recurrence score, and adjuvant therapy decisions.
The Zeiss Intrabeam radiotherapy system provided IORT with 50 kV of low energy X-rays in a single 20 Gy fraction of radiation to the lumpectomy cavity over 17.3–45.27 minutes (mean: 22.26 minutes). The treatment was delivered after sentinel lymph node biopsy was performed and intraoperative assessment confirmed negative results.
The Oncotype DX Breast Cancer Assay (Genomic Health®) was used to assess the likelihood of recurrence. Based on tumor genetics, patients were stratified into high (>31), intermediate [18–30] and low (<18) risk for distant recurrence. The test was ordered at the discretion of the treating physician at the postoperative visit.
Statistical analysis
Descriptive statistics including frequencies (and percentages) for categorical data and means and standard deviations for numerical data were reported. t-test or ANOVA F-tests was used to examine the relationship between the response variable RS (continuous variable) and tumor grade, tumor stage and tumor type (categorical variables). The outcome variable RS was categorized and chi-square test or Fisher’s exact test was used to investigate the relationship between independent variables including grade, stage and tumor type. Multiple linear regression analyses were used to investigate the relationship between the outcome RS (continuous) and explanatory variables: grade, stage, tumor type, adjusting for selected covariates. A P<0.05 was considered statistically significant.
Results
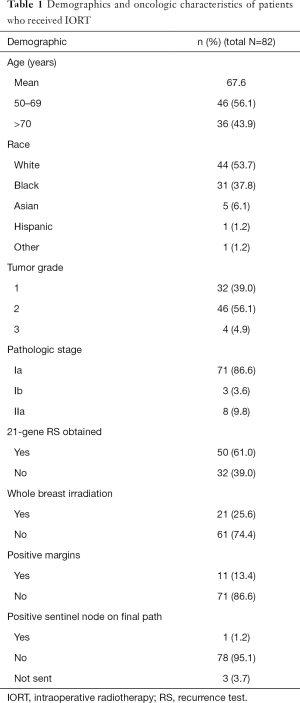
A total of 82 patients were identified who received BCT and IORT. Demographic information of the entire sample is listed in Table 1. The mean age of this group was 67.6 years. Forty-four (53.7%) were Caucasian, 37.8% Black, and 6.1% Asian. In the group as a whole, 95.1% of the patients had a grade 1 or 2 tumor. Eighty-six percent of the patients in the study had a stage I cancer. Of the 82 patients treated with IORT, 61.0% (n=50) had 21-gene recurrence scores evaluated (Table 2). Of these fifty patients, 72% (n=36) were determined low risk for recurrence and 28% (n=14) deemed intermediate risk. There were no patients in the high-risk group.
Full table

Full table
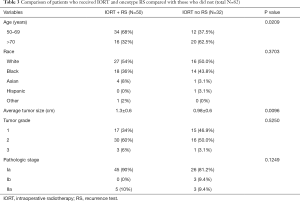
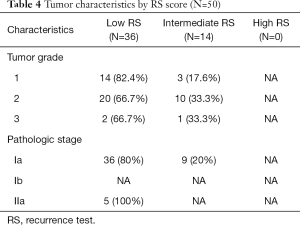
Table 3 lists differences between those who had RS and those who did not. Of the patients with recurrence scores obtained, 90% were stage Ia. Patients over age 70 years were less likely to have RSs ordered. The patients who had RS sent had a larger tumor size than those who did not receive testing (P value <0.05). Of those that had RS sent, 47 of 50 patients (94%) had grade 1 (n=17) or 2 (n=30) tumors. It was noted that 14 out of 17 (82.4%) patients with grade 1 tumors had RS scores less than 18. While 20 out of 30 (66.7%) patients with grade 2 tumors had RS less than 18. Three patients had grade 3 tumors with 2 (66.7%) having a low RS, as shown in Table 4. These results show a trend toward correlation between grade and recurrence score. However, the small number of patients in the sample likely lead to the result being statistically insignificant when examined with linear regression. Thirty-six of 45 patients with stage 1a cancer (80%) had RS less than 18. Linear regression was also used to compare recurrence score with tumor stage; however, no statistically significant correlation was noted.
Full table
Full table
Twenty-one patients (25.6%) in total went on to WBI. Sixteen of these patients had an Oncotype score sent and 5 did not. Eleven patients (13.4%) in total had positive margins, which required re-excision. One patient had a positive sentinel lymph node on final pathology. More patients underwent WBI than the number with positive nodes and margins because patients treated at WHC received IORT as a boost. All patients were offered adjuvant endocrine therapy. In the group with RS, 9 patients were offered chemotherapy due to their score. Two patients went on to have chemotherapy before endocrine therapy. As of May 2016, there has been one recurrence in the study population. This patient did not have RS sent.
Forty-three percent of all patients who received IORT had no RS obtained. The majority of patients did not have documentation in the medical record citing a reason for not testing (n=21). For the patients with documented explanation for not sending RS, the reasons included: documented low index of suspicion of recurrence risk due to small tumors and favorable histology (n=8). “Favorable” tumor features n=5 included 4 patients with mucinous histology and 1 with papillary carcinoma. Three patients were documented to have a poor performance status and one had insufficient specimen for testing.
Discussion
In this study, we report on the first consecutive cases of IORT performed at MGUH and MWHC over a 2-year period. Seventy-two percent of patients who met strict clinical criteria for IORT also had recurrence scores in the low range. Thus, we show that low risk patients, deemed so by strict IORT selection criteria, are also biologically low risk as predicted by the established parameters of the Oncotype DX (Genomic Health®) 21-gene assay. Taking RS into consideration allows one to evaluate whether those determined to be low risk, and consequently deemed candidates for IORT, truly represent a population of patients with favorable tumor biology.
Despite stringent selection criteria for IORT, our data showed a variety of recurrence scores, with almost one third of our patients (28%) having RS in the intermediate range between 18–30. From the validated Oncotype DX literature, this correlates to an average risk of 14.3% for distant recurrence vs. 6.8% risk in the low risk group (17). Out of 14 intermediate risk patients, nine were offered chemotherapy and two went on to receive treatment. Currently, the 21-gene recurrence assay provides a way to clinically assess tumor biology and gauge responsiveness to chemotherapy (18). A low RS confirms a favorable tumor while an intermediate score may lead to vigilant surveillance and possibly the addition of chemotherapy. Therefore, not all “favorable” IORT patients can be assumed to be low risk, permitting omission of systemic treatment beyond hormonal manipulation.
The RS is helpful in guiding subsequent adjuvant treatment, but whether it can be beneficial in guiding local therapy is unknown. Recently, Mamounas et al. examined the relationship between 21-gene recurrence score and locoregional recurrence (19). They concluded that in ER positive patients treated with Tamoxifen, locoregional recurrence was significantly associated with 21-gene RS scores showing a 10-year estimate of local recurrence 4.3% in score <18, 7.2% in those with intermediate scores and 15.8% in those with high scores. Thus, the 21-gene assay may play a role in determining patient selection for IORT based on elevated risk of local recurrence if the RS is intermediate or high. Whether in the preoperative or postoperative setting, the RS can potentially help in determining whether IORT is appropriate in itself or, if given, should be supplemented with whole breast irradiation.
In this study population, the utilization of the RS score was associated with larger tumor size, and patients who were less than 70 years of age (Table 2). While age was not a reason cited by providers for those who did not have testing ordered, we show that the RS was less likely to be ordered in patients age 70 and over. Patterns and preferences of ordering the 21-gene assay in older women are controversial. Studies show that as utilization has increased over the years (20,21), the receipt of the 21-gene recurrence score assay appeared to be inversely associated with age and HER-2 positive status. In addition, Enwold et al. found in a multivariate analysis, older age (age 70+ vs. <50 and HER2+ status were associated with not receiving the 21-gene recurrence score assay, which is consistent with our findings (22).
There are several limitations in this study. First, we acknowledge our small sample size. We attribute this to the recent availability of this technology to our breast program and our strict selection criteria for the delivery of IORT. Furthermore, those patients that have 21-gene RS are selected based on the provider and patient agreement on the utility of the results and whether chemotherapy will be tolerated if necessary. The clinical significance of the RS in IORT patients cannot truly be assessed until long-term recurrence data has been analyzed. Larger numbers with inclusion of adjuvant therapies administered, along with both physician and patient preferences regarding these treatments is needed for this patient population.
Conclusions
Despite these limitations, we explore the idea that it is important to determine whether patients offered IORT indeed have favorable biology given the limited long-term clinical outcome and safety data of this technique (23). The 21-gene assay is one option to determine genomic or tumor profile that is used at our institution. We found no significant correlation between RS and tumor grade or size, which are factors used in the preoperative clinical determination for IORT use. We cannot assume that using standard clinical histopathologic characteristic alone is sufficient as selection criteria given that one third of “safe patients” were found to have an intermediate RS. Genomic testing may provide additional assurance that IORT as a PBI technique is being offered to those who are appropriately low risk. Future directions will seek to evaluate long term follow up data to assess disease free survival, distant and locoregional recurrence in this patient population especially among those with intermediate recurrence scores.
Acknowledgements
None.
Footnote
Conflicts of Interest: Poster was presented at American Society of Breast Surgeons (ASBS), Annual Meeting 2015; April 29–May 03, 2015; Orlando, FL, USA.
Ethical Statement: Institutional Review Boards at both institutions approved the study (No. 20141283).
References
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143-50. [Crossref] [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Curran D, van Dongen JP, Aaronson NK, et al. Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: results of EORTC Trial 10801. The European Organization for Research and Treatment of Cancer (EORTC), Breast Cancer Co-operative Group (BCCG). Eur J Cancer 1998;34:307-14. [Crossref] [PubMed]
- Powell S, Cooke J, Parsons C. Radiation-induced brachial plexus injury: follow-up of two different fractionation schedules. Radiother Oncol 1990;18:213-20. [Crossref] [PubMed]
- START Trialists' Group, Bentzen SM, Agrawal RK, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol 2008;9:331-41. [Crossref] [PubMed]
- START Trialists' Group, Bentzen SM, Agrawal RK, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008;371:1098-107. [Crossref] [PubMed]
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086-94. [Crossref] [PubMed]
- Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513-20. [Crossref] [PubMed]
- Esposito E, Anninga B, Harris S, et al. Intraoperative radiotherapy in early breast cancer. Br J Surg 2015;102:599-610. [Crossref] [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [Crossref] [PubMed]
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. [Crossref] [PubMed]
- Nguyen MT, Stessin A, Nagar H, et al. Impact of oncotype DX recurrence score in the management of breast cancer cases. Clin Breast Cancer 2014;14:182-90. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer version 2.2015. J Natl Compr Canc Netw 2015;13:448-75. [Crossref] [PubMed]
- Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007;25:5287-312. [Crossref] [PubMed]
- Genomic Health delivers the promise and value of precision medicine with the Oncotype IQ portfolio of genomic tests. Accessed June 24, 2016. Available online: http://www.oncotypeiq.com/en-US
- Hudis CA. Biology before anatomy in early breast cancer--precisely the point. N Engl J Med 2015;373:2079-80. [Crossref] [PubMed]
- Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol 2010;28:1677-83. [Crossref] [PubMed]
- Haas JS, Liang SY, Hassett MJ, et al. Gene expression profile testing for breast cancer and the use of chemotherapy, serious adverse effects, and costs of care. Breast Cancer Res Treat 2011;130:619-26. [Crossref] [PubMed]
- Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol 2012;30:2218-26. [Crossref] [PubMed]
- Enewold L, Geiger AM, Zujewski J, et al. Oncotype Dx assay and breast cancer in the United States: usage and concordance with chemotherapy. Breast Cancer Res Treat 2015;151:149-56. [Crossref] [PubMed]
- Barry M, Sacchini V. Evaluating the role of intra-operative radiation therapy in the modern management of breast cancer. Surg Oncol 2012;21:e159-63. [Crossref] [PubMed]


