Breast reconstruction of an unusual configuration using two paranemic implants
Introduction
Implant-based breast reconstruction is widely used because of its less-invasive nature. A ready-made breast implant is usually chosen from implants of various types according to the breast configuration. However, breast shapes that are unsuitable for implant-based breast reconstruction are occasionally encountered. For these cases, implants that account for short height, very long width, and low projection are unavailable. Furthermore, to our knowledge, no case of breast reconstruction with an unusual configuration using multiple implants has been reported. Here, we present two cases of breast reconstruction for patients with wide trunks using a latissimus dorsi myocutaneous flap and two paranemic implants.
Case presentation
Case 1
A 40-year-old woman underwent breast reconstruction using a tissue expander (Allergan, Dublin, Ireland; SX-16, volume: 800 mL, height: 13.5 cm, width: 15.5 cm, projection: 8.1 cm), immediately after total mastectomy. Six months after full expansion with saline (830 mL), we scheduled an expander replacement surgery. Breast reconstruction using a muscle-sparing transverse rectus abdominis myocutaneous flap was not planned; in addition, her breast was too large for reconstruction using either a sole latissimus dorsi myocutaneous flap or a sole implant. Therefore, we performed breast reconstruction using both the latissimus dorsi myocutaneous flap and implant.
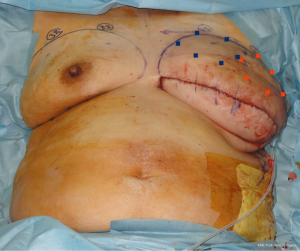
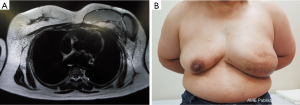
A skin incision was made at a site symmetrical to the inframammary fold of the contralateral breast. The tissue expander was removed and the pedicled latissimus dorsi myocutaneous flap was transferred to the lower portion of the breast defect (Figure 1). The upper portion of the breast defect was measured with a ruler and ultrasonography (height: 9.0 cm, width: 19.0 cm, projection: 3.5–4.0 cm). One implant was inserted into the subpectoral muscle, however, the medial defect could not be reconstructed at this time because of the lack of an appropriate implant. One week later, it was reconstructed with another implant under local anesthesia. A suction drain was inserted and two paranemic implants were appropriately placed (Figure 2). The postoperative course was uneventful. Defatting of the latissimus dorsi myocutaneous flap was performed once and the reconstructed breast showed good results without visible gaps between both implants. Postoperative images showed no abnormal findings (Figure 3).
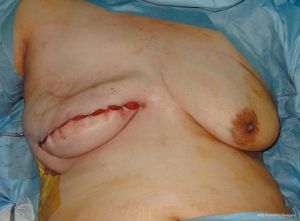
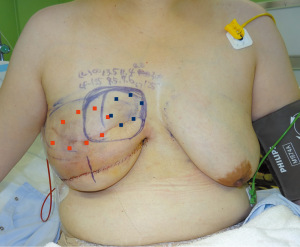
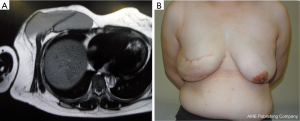
Case 2
A 45-year-old woman underwent breast reconstruction using a tissue expander (Allergan, Dublin, Ireland; SX-16, volume: 800 mL, height: 13.5 cm, width: 15.5 cm, projection: 8.1 cm) immediately after total mastectomy. Seven months after full expansion with saline (980 mL), we scheduled an expander replacement surgery. Similar to case 1, breast reconstruction using a muscle-sparing transverse rectus abdominis myocutaneous flap was not planned. Moreover, her breast was also too large to be reconstructed using either a sole latissimus dorsi myocutaneous flap or implant. Therefore, we performed breast reconstruction using both the latissimus dorsi myocutaneous flap and implant.
A skin incision was made at a site symmetrical to the inframammary fold of the contralateral breast. The tissue expander was removed and the pedicled latissimus dorsi myocutaneous flap was transferred to the lower portion of the breast defect (Figure 4). The upper portion of the breast defect was measured with a ruler and ultrasonography (height: 7.5 cm, width: 25.0 cm, projection: 2.0–2.5 cm). Because of the unavailability of a single suitable implant, the breast was reconstructed using two paranemic implants in the subpectoral muscle; one implant for the lateral defect and the other for the medial defect. A suction drain was inserted and two paranemic implants were appropriately placed (Figure 5). The postoperative course was uneventful. Defatting the latissimus dorsi myocutaneous flap was performed once and the reconstructed breast showed a good result without visible gaps between the implants, although further lateral defatting of the flap was needed. Postoperative images showed no abnormal findings (Figure 6).
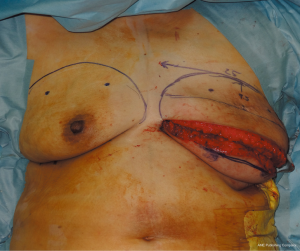
Discussion
Formerly, breast implants were chosen according to the texture (smooth or textured), shape (round or anatomical), hardness, and balance between height and width (height > width, height ≈ width, and height < width). Subsequently, they were chosen based on three linear parameters (height, width, and projection) and volume. There are various breast implant types; however, cases of breast shapes that are unsuitable for implant-based breast reconstruction occasionally occur; breasts with considerable heights and widths, lower projections, large and weighing more than 500 g, with severe ptosis of the contralateral breast (1,2), and cases like ours with a considerably short height, large width, and low projection. In our case, the unusual configuration was successfully reconstructed by two paranemic implants without visible gaps between them as the skin envelope was thick.
The longer the period after the placement of an implant, the greater the probability of its rupture, which may occur six to seven years later, with an even greater probability of rupture after 10 years (3,4). Mechanisms of implant rupture include damage from surgical instruments, shell swelling, fold flaw, or trauma to the implant (5). In a study, 50–64% of implant ruptures were due to damage from surgical instruments (6). Manufacturers do not recommend multiple implant insertion due to the unconfirmed safety of this procedure and the possibility of rupture from friction (7). In principle, implant surgery should be performed with only one implant; however, in our case, obtaining a single implant which was suitable for the defect was challenging; therefore, we performed reconstruction using two paranemic implants. We obtained informed consent from our patients and clarified the postoperative course, the need for regular follow-up with imaging, and the possible removal of the implants in the event of rupture. Although long-term follow-up is needed, we achieved good results for breast reconstruction involving an unusual configuration using a latissimus dorsi myocutaneous flap and two paranemic implants.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Tomita K, Yano K, Nishibayashi A, et al. Aesthetic outcomes of inframammary fold recreation in two-stage, implant-based, breast reconstruction. Springerplus 2016;5:1656. [Crossref] [PubMed]
- Handel N, Jensen JA. An improved technique for creation of the inframammary fold in silicone implant breast reconstruction. Plast Reconstr Surg 1992;89:558-62. [Crossref] [PubMed]
- Hedén P, Nava MB, van Tetering JP, et al. Prevalence of rupture in inamed silicone breast implants. Plast Reconstr Surg 2006;118:303-8; discussion 309-12. [Crossref] [PubMed]
- Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg 2003;138:801-6. [Crossref] [PubMed]
- Hillard C, Fowler JD, Barta R, et al. Silicone breast implant rupture: a review. Gland Surg 2017;6:163-8. [Crossref] [PubMed]
- Handel N, Garcia ME, Wixtrom R. Breast implant rupture: causes, incidence, clinical impact, and management. Plast Reconstr Surg 2013;132:1128-37. [Crossref] [PubMed]
- Allergan. NATRELLE® Silicone-Filled Breast Implants and NATRELLE INSPIRA™ Breast Implants. Available online: https://www.allergan.com/miscellaneous-pages/allergan-pdf-files/l034-03_silicone_dfu


