Two-stage thyroidectomy in the era of intraoperative neuromonitoring
Introduction
In 1933 Prioleau wrote, “a nerve if seen is injured” (1), but a few years later [1938] Lahey supported that, “there was a decrease in the number of injuries of the recurrent laryngeal nerve”, concluding his thoughts after a 3,000 patient series results with standardized dissection and visual identification of the inferior laryngeal nerve (2). Riddell, 30 years later reiterated that Lahey’s opinion and nowadays the visual identification of the recurrent laryngeal nerve (RLN) is considered the gold standard procedure in thyroid surgery (3,4).
As thyroid surgery evolves, nowadays, the use of intraoperative neuromonitoring (IONM) has become widely accepted as a valuable adjunct to the gold standard method of visual identification and dissection of the RLN (5,6). The debate of whether the use of IONM can lead to decreased numbers of RLN palsies still exists and troubles numerous authors and specialists worldwide (5,7-19). There is no proof, statistically at least, of reduced incidence in RLN palsies, in the current literature, with the use of IONM versus visual identification alone. This might be because RLN palsy is a rare event, yet the number of nerves that must be examined to get a statistically significant result is huge (20). There are only few studies supporting the decrease in RLN palsy (transient) when using IONM compared to visual recognition alone, while the sample, for permanent palsy, was small to achieve a statistically significant result despite the decreased trend that it has also shown (8,17). Other large national studies (i.e., Scandinavian) showed contradictory results on the subject (21,22). Therefore, it is not yet safe to come to a clear conclusion.
Recently, in 2011, a group of prominent endocrine surgeons and experts from around the world along with the International Neural Monitoring Study Group (INMSG) proceeded with an international standards guideline statement regarding standardization of the IONM procedure (6). In their statement, the INMSG, includes recommendations regarding standards of equipment, in anesthesia, of equipment setup and endotracheal tube placement, in intraoperative loss of signal (LOS) evaluation and finally in waveform definition and assessment. The above represents a valuable “manual” for those fond of the IONM thyroid surgery and helps, through standardization of the procedures, to establish a global “language” regarding IONM and minimize the possibility of any bias (6,23).
RLN palsy is still considered a significant complication in thyroid surgery. Even in experienced hands, transient unilateral RLN palsy can be up to 9.8% when referring to first operations and up to 12.5% when referring to reoperations (2,24-28). “High-risk” patients—malignancy, retrosternal/mediastinal large goiters or Graves disease—seem to carry a higher risk for this on the one hand but they altogether represent the majority of the surgical indications in thyroid surgery (27). A more fearful and sometimes life-threatening situation is bilateral RLN palsy even if transient. Not only vocal dysfunction and physical limitation but also airway obstruction threat makes this complication, even if extremely rare (0.1–0.9%), potentially lethal for the patient (5,29,30).
Recently, INMSG and other authors suggested a different approach when RLN palsy occurs in the first side of dissection, naming this a staged or two-stage thyroidectomy. What they suggest is that when there is a LOS on the first side of dissection, and after verification of the event following the INMSG proposed steps, the procedure should stop until full nerve recovery is completed at a later stage when the vocal cord intact functionality is established with laryngoscopy (6,23,30-33).
Following this recommendation, we have been applying staged thyroidectomy in our Department for the last 4 years. Patients with a LOS on the first side were followed up with scheduled laryngoscopy and safely proceeded to the completion surgery when the VC functionality recovered. Our results are presented here.
Methods
Our aim was firstly to determine the incidence of signal loss on the first side of resection. Secondly to present how our operative strategy changed due to this knowledge combined with the results of postoperative laryngoscopy. Thirdly to analyze the different approach (if any) in patients with benign or malignant disease and finally to show how this change in operative strategy altered our results in temporary and permanent vocal cord palsy (VCP).
We carried out and present here a retrospective, observational cohort study of prospectively collected data from all patients who underwent a scheduled total thyroidectomy with or without neck dissection in Endocrine Surgery Department of Central Clinic of Athens the last 4 years. The institution’s Ethical Board Committee approved the study and all patients have signed the informed consent form.
The inclusion criteria involved all patients planned for total thyroidectomy with or without neck dissection. The exclusion criteria, on the other hand, were previous neck operation, parathyroid surgery, pre-existing VCP and unilateral surgery.
All patients were preoperatively informed, among others, during the consent about the possibility of stage procedure and how this would differentiate the postoperative period and planning of their treatment. Any questions raised by the patient were resolved preoperatively in a multidisciplinary group consisting of the patient’s referring endocrinologist, associate ENT specialist and the Endocrine Surgery team members. Preoperative indirect laryngoscopy (L1) was mandatory and essential for all patients, while all patients also underwent the same procedure, by an experienced ENT specialist team, on the 1st or 2nd postoperative day (L2). Those with confirmed VCP were followed up with laryngoscopy on the 2nd, 4th and 6th postoperative month, to confirm or exclude recovery of the affected side. Permanent vocal cord paralysis was defined when it persisted for more than 6 months.
Expert anesthesiologists intubated patients with either a NIM electromyography (EMG) endotracheal tube (Medtronic, Jacksonville, FL, USA) or an electrode tube (AVALANCHE® SI System, Dr. Langer Medical GmbH, Waldkirch, Germany). There were strict instructions not to use any long-acting muscle relaxant agents at any point of the procedure. The algorithm for monitoring tube placement intubation, proposed by INSMG, was followed throughout the process.
IONM procedure followed the INMSG Guidelines Statement published in 2011. Surgery began from the dominant side, which could be the side with the suspicious or malignant nodule, the bigger nodule or the greater number of nodules. If neither of the previous was present on the first side of resection, it was the left one. After a slight mobilization of the lobe, a vagus signal (V1) was obtained with stimulation of 1–2 mA. In the absence of a positive response to the stimulus, the proposed algorithm was carried out to exclude malpositioning of the endotracheal tube or possible remaining effect of muscle relaxants. The next step was RLN mapping through stimulation with 1–2 mA, before visual identification. After the positive response (R1), a full nerve dissection and visual identification was performed, while nerve integrity was evaluated with repeated stimulation throughout lobe mobilization and removal. Once the first side of resection was completed, both RLN and vagus nerve response after stimulation was required to proceed to the other side (R2, V2). The same procedure was carried out on the opposite side of the thyroid gland (R1, V1, R2, V2), and once the thyroidectomy was completed positive responses from both RLN and vagus nerve were recorded after stimulation on the first side of resection (R3, V3). All the stimulation results were electronically registered and saved in a storage device, while they were also printed on paper for documentation and future use or reference. A positive response to nerve stimulation, in all the previous steps, was considered when the peak altitude of the stimulation curve was over 100 µV. In cases of a significant drop or LOS on the first side of the resection, as this was established following the standards in intraoperative LOS proposed by INMSG, the lead surgeon on the team took the decision of whether or not to proceed to the contralateral lobe (6).
The complete absence of stimulation curve after nerve stimulation with 1–2 mA was defined as LOS, while a significant drop in the signal was considered when there was a decrease in response to nerve stimulation greater than 50% of the initial value. In cases with LOS or significant drop of the signal, there were 10–30 min intermissions before re-evaluation of nerve response. If the absence of a response or the decrease remained, nerve injury was highly suspected, and the lead surgeon took the decision to proceed or stage the initially planned operation. Whatever the decision was, there was a complete description and photographic documentation of the incident in the patient’s operative report.
The suspected injury was categorized as segmental (type 1) or global (type 2), as this was proposed by INMSG, in a multicenter (POLT) study published in 2016 (34). In all patients, the RLN was thoroughly dissected and exposed by the surgeon to verify its anatomical integrity throughout its course. Segmental lesion (type 1) was considered in cases with complete LOS peripherally from a particular point of the RLN’s course, while the centrally (towards the larynx) to this point signal was intact. Global injury (type 2), on the other hand, was considered in the complete absence of a response to 1–2 mA stimulation throughout RLN and vagus nerve’s course. Late recovery time and more severe injuries were reported in type 1 compared to type 2 injury (34).
The same experienced ENT specialist team subjected all patients to laryngoscopy the 1st or 2nd postoperative day, to evaluate the vocal cord (VC) mobility and proceed to the follow-up planning. If the VCP was confirmed, patients were informed accordingly and scheduled for re-evaluation on the 2nd, 4th and 6th postoperative month. During this period, and when the VC mobility recovered patients were scheduled for completion thyroidectomy following the stage procedure proposed. In all cases, the completion thyroidectomy was carried out at least three months postoperatively. In those cases that the nerve recovered immediately and the postoperative laryngoscopy revealed normal VC mobility, we proceeded to completion thyroidectomy on the 1st or 2nd postoperative day.
Results
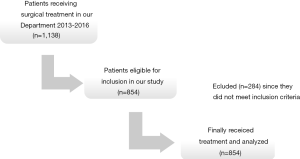
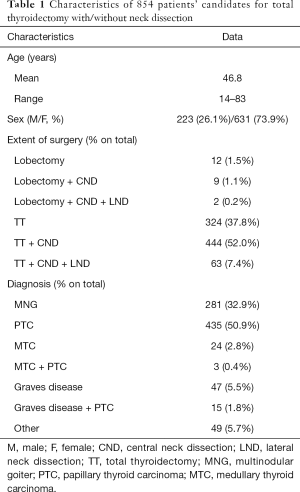
From January 2013 to December 2016, a 4 years period, 1,138 patients were treated surgically in our Department. Of those patients, 854 were candidates for total thyroidectomy with/without neck dissection and were eligible for inclusion in our study. Flowchart of the patients is shown in Figure 1. There were 631 women and 223 men, with a median age at the time of surgery 46.8 (range 14–83) years. The patients’ clinical characteristics are presented in Table 1.
Full table
We experienced 72 VCP; 2 of those were due to intentional resection of the RLN because of tumor invasion. Hence, we ended up with 70/854 (8.2%) patients with VCP. Of those patients 34 had LOS on the first side of resection (Group 1), 22 experienced the event on the second side of resection (Group 2), and in 14 cases there was VCP on the postoperative laryngoscopy (L2) without signal loss or significant (>50%) decrease from the initial recorded value (Group 3). Patients from Groups 2 and 3 were not analyzed for this study’s purposes and therefore no further interpretation serves this study’s aim. Based on IONM and following our scheduled protocol, 23 of the Group 1 patients were staged and included in our study. The rest of the Group 1 patients underwent total thyroidectomy with/without neck dissection for different reasons. In four of those cases, patients underwent total thyroidectomy with central neck dissection since the first side of resection LOS was only documented at the final evaluation of nerves functionality (R3, V3), possibly due to contralateral lobe traction. The remaining seven cases included four patients with an extensive malignant disease and nodal involvement, capsular invasion or/and multifocality of the tumor. Therefore the leading surgeon took the decision to proceed to a total thyroidectomy with central or/and lateral neck dissection wherever needed. The remaining three cases consisted patients where the response, after 2 mA vagus nerve stimulation and 20 min intermission after the event, was at least >100 µV. Up to that point of time, the team’s belief was that when the response is over 100 µV, the nerve is functionally integral. An opinion that changed as experience in our Department increased, and even in the international literature decrease from the initial value greater than 50% is a more accurate indication of nerve injury (35-38). It is worth mentioning that all the seven cases that had a signal loss during the first side of resection and underwent total thyroidectomy with neck dissection occurred during the first year of our new operative strategy implementation.
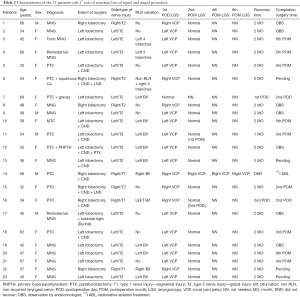
Twenty-three patients, initially planned for total thyroidectomy were staged according to IONM results. The patients’ characteristics, i.e., indication for surgery, side of resection, RLN variation, recovery time, are presented in Table 2. In 22 patients the VCP was transient and RLN functionality fully recovered as the 2nd postoperative month laryngoscopy revealed. One patient is found to have permanent VCP since the RLN did not recover even after the 6th postoperative month laryngoscopy.
Full table
The 2nd postoperative month laryngoscopy and the first patient underwent completion surgery (papillary carcinoma) 3 months after the initial operation, while the other is scheduled for completion surgery the next month (suspicious contralateral nodule). The patient with the permanent RLN injury, after receiving a detailed consultation regarding the dangers of a possible completion surgery decide not to receive surgical treatment and therefore, in agreement with his attending endocrinologist, he received iodine radioactive treatment that successfully destroyed the thyroid remnant. The patient’s follow-up shows untraceable thyroglobulin and negative ultrasonography one and half year after the treatment.
In 17 cases of the staged procedure, the first side of resection was left, while in 6 patients the first side was the right. More than half of the staged cases (14/23, 61%) involved a RLN variation—11 bifurcations, 1 case with four branches and 2 cases with three branches, while in one of them a non-RLN coexisted. All the above variations were documented with intraoperative photographs and videos for future reference, after, of course, the preoperative informed consent of all the patients. Regarding the patients’ pathology, 10/23 (43.5%) patients had papillary or medullary thyroid cancer whereas the remaining 13/23 (56.5%) patients had a multinodular/toxic goiter.
Only in one case, with signal loss, the 1st postoperative day laryngoscopy showed normal VC mobility. It can be explained by either an immediate full nerve recovery or by false positive IONM signal loss. The false positive explanation shows more probable. Whatever the case was, the informed patient underwent completion thyroidectomy safely the 2nd postoperative day, and VC normal function was established again during the 2nd postoperative laryngoscopy. This result strongly supports our decision for change in operative strategy, since in 22/23 cases (PPV >95%) of signal loss the nerve injury was confirmed by the next day laryngoscopy.
For the record, in all 70 patients with signal loss or a significant decrease in its value, the affected nerve was routinely exposed to its entire course in the groove and the nerves’ anatomical integrity was visually identified and photographically documented.
Discussion
The use of IONM in thyroid surgery has gradually become a widely accepted adjunct in thyroid surgery. It is the only method that provides the surgeon intraoperative information regarding nerve’s identification and functional integrity. Therefore, it supplements the gold standard method of visual identification with valuable information about nerves anatomical integrity and functional ability. Whether its use decreases the RLNP incidence still remains controversial (5,7-19,39-41).
One of the most fearful complications in thyroid surgery is bilateral VCP due to nerve injury (42). A complication that, according to statistics, even the most expert surgeons in the field might experience during their career (5,29). The international literature throughout the years supports field expertise, experience and visual identification of the RLN as the cornerstones of RLN’s anatomical and functional integrity (30,32). We present here our series after the implementation of two-stage thyroidectomy with IONM in our Department. With the implementation of this strategy, we can preserve a patients’ quality of life by not putting them in unnecessary intraoperative risks due to minimization of the risk of bilateral RLN to nearly 0%. Other results by authors support our decision for an operative strategy change (23,30-33,43). Dralle et al., in a recently published study, demonstrated that in Germany nearly 90% of surgical departments could use IONM in thyroid procedures. The most impressive finding from this study was that more than 90% of the surgeons were keen to change their operative strategy, to a two-stage process, to avoid bilateral nerve injury (23). In addition to that, Goretzki et al., mention that in their series when the signal loss on the first side of resection did not change the operative strategy from the initially planned total thyroidectomy, the risk for bilateral nerve injury increased to 19% (30).
As other authors demonstrated, in many cases a visually intact nerve can be at the same time a non-functional nerve (20,21,23,30). Bergenfelz et al., in a multicenter study, showed that only a few injured nerves, 11.3% for unilateral and 16.7% for bilateral lesions, can be visually identified intraoperatively (21). This fact is emphasized and by two German publications, by Goretzki et al. and Dralle et al., which support that the use of IONM is the most reliable method to identify nerve injury in an anatomically intact nerve (20,30). In our series, only 4/70 (5.7%) patients had a visually detectable injury of the impaired RLN. Three of them had transient VCP, while the fourth had permanent unilateral VCP.
Based on INMSG Guidelines Statement published in 2011 regarding IONM and our growing experience throughout the years we applied two-stage thyroidectomy in 23 cases over the last 4 years. Through this implementation, we experienced zero cases of bilateral RLNP, and patients’ satisfaction level remained unchanged despite the different approach to their treatment process. International literature reveals that IONM use in thyroid surgery shows a high negative predictive value (NPV), as a standardized method, which varies from >90% to 100% of the cases. On the other hand, though, its positive prognostic value (PPV) shows a huge range of values that lies from 10% to 90% (33). The primary explanation is the significant number of false positive results that many authors suggest, and therefore question this IONM use (9,44). In their Guideline Statement, INMSG proposes an algorithm—intraoperative LOS evaluation standard—that provides sufficient assistance and “guidance” to the surgeon to exclude or confirm a possible signal loss and plan the next step of the procedure (6). This algorithm application, according to authors, can raise the percentages of variable PPV up to 75% and in that way make IONM a reliable adjunct to thyroid surgery. In our series, we experience only one case of possible false positive LOS (1/23, 4.34%) and we attribute this low incidence to our strict adherence to the INMSG’s recommendations.
Something that troubled us and we believe that it should be the subject of future research on the field was the fact that we experienced 14 cases (14/70, 20%) with false negative IONM. Twelve of those patients had transient unilateral VCP, one of those has persistent unilateral VCP 4 months after total thyroidectomy and the last waiting for the first re-evaluation of VC mobility 2 months after her surgery. INMSG in their 2016 meeting in Boston, USA, presented their preliminary results from a multicenter prospective study, for Identification of False Negative causes in EMG monitored thyroid surgery, called IFAN. Analyzing or commenting on these results is beyond the scope of the present study. Authors emphasize the importance of false negative results of IONM, since “it enhances patient’s risk of bilateral RLNP” (45).
Another intriguing observation is that we experienced four cases with signal loss and consequently VCP on the contralateral site of the surgery. In other words, we experienced four cases with signal loss at the final check (R3, V3) on the first side of resection while the signals after the lobectomy completion (R1, V1) were intact.
Some other studies, on the other hand, suggest that a mild intraoperative injury of the nerve that causes a signal decrease might recover intraoperative if the surgeon makes an intermission (44). Our experience showed that this recovery is not always reliable and that not enough experience has yet been attained on the subject. As previously reported, we had seven patients with the first side of resection LOS that eventually underwent total thyroidectomy (finally all of them had unilateral transient VCP on the 1st postoperative day laryngoscopy). In four of them, the decision was made because of the extensive disease they suffered and the peculiar and “fragile” mentality of those patients at the time of surgery. In the other three patients, we experienced “nerve recovery” intraoperative, with vagus signals >100 µV, and thus we decided to proceed to bilateral surgery. All three patients had unilateral VCP on the 1st postoperative day laryngoscopy, and therefore we reconsider our opinion regarding “nerve recovery” and eventually abandon this tactic.
Another issue pointed out by INMSG on their Guideline Statement is the importance and need for both pre- and post-thyroidectomy laryngoscopy in all patients and not only in those considered as “high risk” (Graves disease, reoperations, substernal goiters) patients (6,27). Voice changes or impairment is not a trusted tool and as many authors support this characteristic can be misleading in several cases (27,46,47). Superior laryngeal nerve injury, intraoperative manipulations (strap muscles division) or even intubation injury may affect patient’s voice without any VCP saw on laryngoscopy. Various series report incidences of 10%, 32% or even 50% of asymptomatic patients with unilateral VCP (6,27,46-48). Therefore laryngoscopy stands as the only useful tool for VC mobility evaluation both preoperatively and postoperatively in all patients undergoing thyroid surgery.
With our limited experience in this newly adopted strategy, there are some key points that someone has to take into account when dealing with monitored thyroid surgery. We suggest the routine pre- and postoperative laryngoscopy to all patients undergoing thyroid surgery since symptoms are not the rule—and even if they have various exceptions. Guide the anesthesiologist appropriately in the correct placement of the tube and discourage them from using long-acting relaxants. Preferably start the surgery on the dominant side—suspicious nodule or malignancy, a greater number of nodules, larger nodules or a bigger lobe (43). Follow the rule of V1, R1, R2, V2 on each side of operation—system’s functionality and nerves integrity are only guaranteed when a positive response from ipsilateral vagus nerve is present. Use the algorithm proposed by the INMSG to exclude or confirm a possible LOS. In the case of signal loss on the first side of resection, it is sensible to reconsider bilateral surgery to avoid the unnecessary risk of bilateral VCP and its sequences in a patient’s quality of life. If the postoperative laryngoscopy confirms the signal loss we advise a close follow-up with laryngoscopy re-evaluation on 2nd, 4th or 6th postoperative month to schedule the completion surgery once the VC recovers. The extreme majority of patients appreciate and acquiesce to the decision for stage operation to avoid any risk of bilateral nerve injury. As Dionigi et al. support to their study, 87% of the affected nerves recover within six months of surgery and 89% of them in 12 months (27).
Despite some limitations in our study, i.e., the retrospective nature of it, we were able to jump into some valuable conclusions that by the existing literature led to modification of our, up to that point of time, operative strategy and minimize the risk of bilateral VCP.
Future larger, possibly multicenter studies in the same direction could verify and enhance our experience. The possible financial cost from this operative strategy and the psychological part regarding the patients’ expectation should also be taken into account in future studies.
Conclusions
RLN visual identification and expertise remain the standard care in thyroid surgery. Routine use of IONM in thyroid surgery, on the other hand, provides the surgeon with the only real time information regarding a nerve’s functional integrity. This knowledge can help the surgeon to reconsider bilateral surgery in cases with signal loss on the first side of resection. For this reason, the risk of bilateral nerve injury is almost zero. By virtually eliminating one of the worst complications of thyroid surgery, staged thyroidectomy seems a very attractive and promising procedure for both patient and surgeon. We recommend stage thyroidectomy in all those cases, even in malignancies, since the benefits are much more than the disabilities in a patient’s morbidity and quality of life.
Acknowledgements
Authors would like to thank Mr. Athanasios Antonopoulos for his valuable assistance with the manuscript’s English language editing.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors confirm that they have obtained ethics approval by the “Scientific Board” of Central Clinic of Athens (approval ID: E001/2016). The authors also confirm that all patients gave their informed consent.
References
- Welbourn RB. The History of Endocrine Surgery. 1st ed. New York: Praeger Publishers, 1990.
- Lahey FH, Hoover WB. Injuries to the recurrent laryngeal nerve in thyroid operations: Their management and avoidance. Ann Surg 1938;108:545-62. [Crossref] [PubMed]
- Riddell VH. The surgery of the thyroid gland. Postgrad Med J 1960;36:447-61. [Crossref] [PubMed]
- Riddell V. Thyroidectomy: prevention of bilateral recurrent nerve palsy: results of identification of the nerve over 23 consecutive years (1946-69) with a description of an additional safety measure. Br J Surg 1970;57:1-11. [Crossref] [PubMed]
- Dralle H, Sekulla C, Lorenz K, et al. German IONM Study Group 2008 Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg 2008;32:1358-66. [Crossref] [PubMed]
- Randolph GW, Dralle H. International Intraoperative Monitoring Study Group, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121:S1-16. [Crossref] [PubMed]
- Hermann M, Hellebart C, Freissmuth M. Neuromonitoring in Thyroid Surgery: Prospective Evaluation of Intraoperative Electrophysiological Responses for the Prediction of Recurrent Laryngeal Nerve Injury. Ann Surg 2004;240:9-17. [Crossref] [PubMed]
- Barczyński M, Konturek A, Cichoń S. Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg 2009;96:240-6. [Crossref] [PubMed]
- Chiang FY, Lu IC, Kuo WR, et al. The mechanism of recurrent laryngeal nerve injury during thyroid surgery - the application of intraoperative neuromonitoring. Surgery 2008;143:743-9. [Crossref] [PubMed]
- Chan WF, Lang BH, Lo CY. The role of intraoperative neuromonitoring of recurrent laryngeal nerve during thyroidectomy: a comparative study on 1000 nerves at risk. Surgery 2006;140:866-72; discussion 872-3. [Crossref] [PubMed]
- Shindo M, Chheda NN. Incidence of vocal cord paralysis with and without recurrent laryngeal nerve monitoring during thyroidectomy. Arch Otolaryngol Head Neck Surg 2007;133:481-5. [Crossref] [PubMed]
- Thomusch O, Sekulla C, Machens A, et al. Validity of intra-operative neuromonitoring signals in thyroid surgery. Langenbecks Arch Surg 2004;389:499-503. [Crossref] [PubMed]
- Randolph GW, Kobler JB, Wilkins J. Recurrent laryngeal nerve identification and assessment during thyroid surgery: laryngeal palpation. World J Surg 2004;28:755-60. [Crossref] [PubMed]
- Sturgeon C, Sturgeon T, Angelos P. Neuromonitoring in thyroid surgery: attitudes, usage patterns, and predictors of use among endocrine surgeons. World J Surg 2009;33:417-25. [Crossref] [PubMed]
- Dionigi G, Bacuzzi A, Boni L, et al. What is the learning curve for intraoperative neuromonitoring in thyroid surgery? Int J Surg 2008;6:S7-12. [Crossref] [PubMed]
- Goretzki PE, Dotzenrath C, Witte J, et al. Chirurgie des Morbus Basedow. Viszeralchirurgie 2000;35:117-23. [Crossref]
- Vasileiadis I, Karatzas T, Charitoudis G, et al. Association of intraoperative neuromonitoring with reduced recurrent laryngeal nerve injury in patients undergoing total thyroidectomy. JAMA Otolaryngol Head Neck Surg 2016;142:994-1001. [Crossref] [PubMed]
- Higgins TS, Gupta R, Ketcham AS, et al. Recurrent laryngeal nerve monitoring versus identification alone on post-thyroidectomy true vocal fold palsy: a meta-analysis. Laryngoscope 2011;121:1009-17. [Crossref] [PubMed]
- Dionigi G, Barczynski M, Chiang FY, et al. Why monitor the recurrent laryngeal nerve in thyroid surgery? J Endocrinol Invest 2010;33:819-22. [Crossref] [PubMed]
- Dralle H, Sekulla C, Haerting J, et al. Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery 2004;136:1310-22. [Crossref] [PubMed]
- Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg 2008;393:667-73. [Crossref] [PubMed]
- Khamsy L, Constanthin PE, Sadowski SM, et al. Loss of neuromonitoring signal during bilateral thyroidectomy: no systematic change in operative strategy according to a survey of the French Association of Endocrine Surgeons (AFCE). BMC Surgery 2015;15:95. [Crossref] [PubMed]
- Dralle H, Sekulla C, Lorenz K, et al. Loss of the nerve monitoring signal during bilateral thyroid surgery. Br J Surg 2012;99:1089-95. [Crossref] [PubMed]
- Hermann M, Alk G, Roka R, et al. Laryngeal recurrent nerve injury in surgery for benign thyroid diseases: effect of nerve dissection and impact of individual surgeon in more than 27,000 nerves at risk. Ann Surg 2002;235:261-8. [Crossref] [PubMed]
- Thomusch O, Machens A, Sekulla C, et al. Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: Prospective multicenter study in Germany. World J Surg 2000;24:1335-41. [Crossref] [PubMed]
- Barczyński M, Konturek A, Pragacz K, et al. Intraoperative nerve monitoring can reduce prevalence of recurrent laryngeal nerve injury in thyroid reoperations: results of a retrospective cohort study. World J Surg 2014;38:599-606. [Crossref] [PubMed]
- Dionigi G, Boni L, Rovera F, et al. Postoperative laryngoscopy in thyroid surgery: proper timing to detect recurrent laryngeal nerve injury. Langenbecks Arch Surg 2010;395:327-31. [Crossref] [PubMed]
- Jeannon JP, Orabi AA, Bruch GA, et al. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: a systematic review. Int J Clin Pract 2009;63:624-9. [Crossref] [PubMed]
- Röher HD, Goretzki PE, Hellmann P, et al. Complications in thyroid surgery. Incidence and therapy. Chirurg 1999;70:999-1010. [PubMed]
- Goretzki PE, Schwarz K, Brinkmann J, et al. The impact of intraoperative neuromonitoring (IONM) on surgical strategy in bilateral thyroid diseases: is it worth the effort? World J Surg 2010;34:1274-84. [Crossref] [PubMed]
- Schneider R, Lorenz K, Sekulla C, et al. Surgical strategy during intended total thyroidectomy after loss of EMG signal on the first side of resection. Chirurg 2015;86:154-63. [Crossref] [PubMed]
- Fontenot TE, Randolph GW, Setton TE, et al. Does intraoperative nerve monitoring reliably aid in staging of total thyroidectomies? Laryngoscope 2015;125:2232-5. [Crossref] [PubMed]
- Sadowski SM, Soardo P, Leuchter I, et al. Systematic use of recurrent laryngeal nerve neuromonitoring changes the operative strategy in planned bilateral thyroidectomy. Thyroid 2013;23:329-33. [Crossref] [PubMed]
- Schneider R, Randolph G, Dionigi G, et al. Prospective study of vocal fold function after loss of the neuromonitoring signal in thyroid surgery: the International Neural Monitoring Study Group’s POLT study. Laryngoscope 2016;126:1260-6. [Crossref] [PubMed]
- Schneider R, Bures C, Lorenz K, et al. Evolution of nerve injury with unexpected EMG signal recovery in thyroid surgery using continuous intraoperative neuromonitoring. World J Surg 2013;37:364-8. [Crossref] [PubMed]
- Schneider R, Randolph GW, Sekulla C, et al. Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 2013;35:1591-8. [Crossref] [PubMed]
- Phelan E, Schneider R, Lorenz K, et al. Continuous vagal IONM prevents recurrent laryngeal nerve paralysis by revealing initial EMG changes of impending neuropraxic injury: a prospective, multicenter study. Laryngoscope 2014;124:1498-505. [Crossref] [PubMed]
- Schneider R, Sekulla C, Machens A, et al. Postoperative vocal fold palsy in patients undergoing thyroid surgery with continuous or intermittent nerve monitoring. Br J Surg 2015;102:1380-7. [Crossref] [PubMed]
- Mangano A, Wu CW, Lianos GD, et al. Evidence-based analysis on the clinical impact of intraoperative neuromonitoring in thyroid surgery: state of the art and future perspectives. Surg Technol Int 2014;25:91-6. [PubMed]
- Chan WF, Lo CY. Pitfalls of intraoperative neuromonitoring for predicting postoperative recurrent laryngeal nerve function during thyroidectomy. World J Surg 2006;30:806-12. [Crossref] [PubMed]
- Wu CW, Hao M, Tian M, et al. Recurrent laryngeal nerve injury with incomplete loss of electromyography signal during monitored thyroidectomy-evaluation and outcome. Langenbecks Arch Surg 2017;402:691-9. [Crossref] [PubMed]
- Brok HA, Copper MP, Stroeve RJ, et al. Evidence for recurrent laryngeal nerve contribution in motor innervation of the human cricopharyngeal muscle Laryngoscope 1999;109:705-8. [Crossref] [PubMed]
- Dionigi G, Frattini F. Staged thyroidectomy: time to consider intraoperative neuromonitoring as standard of care. Thyroid 2013;23:906-8. [Crossref] [PubMed]
- Sitges-Serra A, Fontané J, Dueñas JP, et al. Prospective study on loss of signal on the first side during neuromonitoring of the recurrent laryngeal nerve in total thyroidectomy. Br J Surg 2013;100:662-6. [Crossref] [PubMed]
- Ferrari CC, Dionigi G, Barczynski M, et al. Prospective Evaluation Study for Identification of False Negative Causes in Electromyographical Monitored Thyroid Surgery (IFAN), INMSG 2016 Meeting, Boston, USA.
- Stevens K, Stojadinovic A, Helou LB, et al. The impact of recurrent laryngeal neuromonitoring on multi-dimensional voice outcomes following thyroid surgery. J Surg Oncol 2012;105:4-9. [Crossref] [PubMed]
- Randolph GW, Kamani D. The importance of preoperative laryngoscopy in patients undergoing thyroidectomy: voice, vocal cord function, and the preoperative detection of invasive thyroid malignancy. Surgery 2006;139:357-62. [Crossref] [PubMed]
- Farrag TY, Samlan RA, Lin FR, et al. The utility of evaluating true vocal fold motion before thyroid surgery. Laryngoscope 2006;116:235-8. [Crossref] [PubMed]