Inadvertent parathyroidectomy risk factors in 1,373 thyroidectomies—male gender and presence of lymphadenopathy, but not size of gland, independently increase the risk
Introduction
Thyroidectomy has been shown to be a safe operation in the hands of experienced surgeons (1). The overall morbidity is very low and mortality nowadays is almost an anecdotal occurrence with many centres discharging patients as early the first post-operative day (2). Transient hypocalcemia caused by inadvertent parathyroidectomy (IP) or disruption of the blood supply to the parathyroid glands (PG) is the most commonly occurring complication of thyroidectomy operations (3). The rate of such transient hypocalcemia presents great variations in the published literature, ranging from 2–53% (4,5). According to the latest report from The British Association of Endocrine and Thyroid Surgeons 26.2–28.6% of patients will need develop post-operative hypocalcaemia after total thyroidectomy (TT) (6). The report has shown that early hypocalcaemia is increased by lower age, female gender, Graves’ disease and level VI dissection (6).
In order to avoid IP, good practise calls for capsular dissection of the thyroid gland, an effort to identify and preserve as many PG as possible and ligation of the inferior thyroid artery close to the thyroid gland avoiding ligation of the branches running to the PG and the ensuing devascularisation. Despite such best efforts though, IP is a well-recognised complication of thyroid surgery (7).
A number of studies in the literature have published conflicting evidence on the factors that influence IP, a result of the small number of patients included, differences in practises between centres and variations in the population included in the studies (5,8-23). The purpose of this study was to evaluate the incidence of IP during thyroid operations in a large single centre case series during a period of 6 years and identify the risk factors involved.
Methods
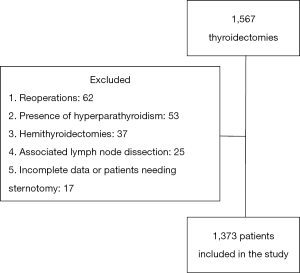
This was a retrospective review of all TT operations performed in a single institution (General Hospital of Athens Polikliniki) from January 2004 to December 2009. All the operations were performed by three consultant endocrine surgeons or by their registrars under direct supervision. Inclusion criteria for the study were all thyroidectomies [TT or near-total thyroidectomies (NTT)] performed during the 6-year period including surgery for thyroid cancer (TC). Exclusion criteria included: all re-operative cases, thyroid operations requiring sternotomy, cases with combined thyroid and parathyroid pathology, hemithyroidectomies and cases involving neck lymph nodes (LN) dissections (central or lateral or both).
Pathology reports were screened to identify inadvertent PG removal. The location (intracapsular, intrathyroidal, extracapsular), number, and size of the removed PG was noted. PG submitted separately for examination were not considered in this study. Operative reports of relevant cases were reviewed to exclude the possibility of intentional removal and the clinical records for the selected patients were accessed for patient demographic data, preoperative diagnosis, and operative details. Patients were grouped as having TT (on both sides), NTT (on both sides) and TL/NTL (total lobectomy on one side and near-total lobectomy on the other side).
Operative and pathology reports were also screened for the presence of LN (number, location, histology) and the presence of TC (type of TC, location, focality, size of largest tumor, invasion of thyroid capsule, extrathyroidal extension and vessel invasion).
The dimensions of the thyroid lobe (longitudinal, transverse and anteroposterior) as documented on the histopathology report were analysed. The volume of each thyroid lobe was calculated according to the following formula: volume = Pi/6 × (longitudinal axis × transverse axis × anteroposterior axis).
Surgical technique was based on the principle of capsular dissection, with every effort made to identify and preserve all four PGs. In case of devascularisation or incidental discovery of a PG on the specimen, an auto-transplantation protocol to the sternocleidomastoid muscle was followed. All patients stay in overnight and are seen 2–4 weeks after their operation for a follow-up appointment. Our protocol calls for a check of serum calcium and parathyroid hormone on the next morning after the operation. We do not commence on calcium supplementation unless they become clinically symptomatic or they have biochemical evidence of hypocalcaemia. The study has been approved by the institutional review board (IRB) of our institution and it conforms to the provisions of in accordance with the Helsinki Declaration as revised in 2013. The study outcomes did not affect the future management of the patients.
Data collection and analysis of the results was performed with adherence to data protection principles. Statistical analysis was done using the SPSS software (SPSS 20, Chicago, IL, USA). Descriptive statistics were expressed as frequencies and percentages for categorical and as means/medians with standard deviations (SD) for continuous variables. To investigate the correlations between IP and our independent variables we used chi-square test, corrected with Fisher’s exact test when appropriate, for categorical and logistic regression for continuous variables. Statistically significant were considered the results with P<0.5. A multivariable logistic regression analysis was performed to determine the independent risk factors for IP. The selection of the variables to be included in this model was based on the statistical significance of the correlation in the univariate analysis and they were entered in the model in a single step using the enter method.
Results
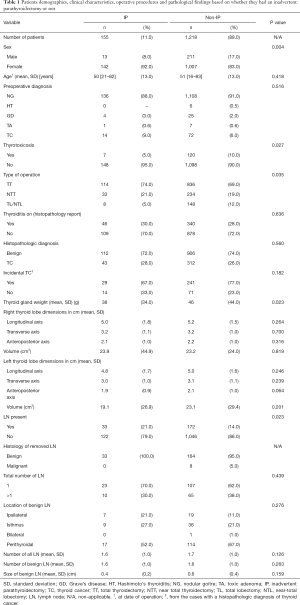
A total of 1,373 patients who underwent thyroidectomy were included in our study, 1,149 of them females (84%). Figure 1 shows a flowchart of the subjects’ selection process in our study. There were 155 patients (11%) that had an IP while the remaining 1,218 patients (89%) did not have an IP after their operation. Table 1 shows the clinical and pathological characteristics of the population in total, as well as in groups of patients that did and did not underwent IP. The univariate analysis showed that gender, thyroid gland weight, thyroid activity pre-operatively and type of operation are associated with the occurrence of IP. There was a significantly higher number of females in the IP group compared to the non-IP group (92% vs. 83%, P=0.004). The mean age of the patients when operated was 50.9 years (range, 16–80 years). The non-IP group had a significantly higher number of thyrotoxic patients (10% vs. 5%, P=0.027) and a significantly higher mean weight of the thyroid gland (46 vs. 38 g, P=0.023) compared to the IP group. The non-IP group had also a significantly lower number of TT and a higher number of TT/NTT compared to the IP group (P=0.035). Table 1 also presents a comparison of thyroid lobes dimensions and volumes between IP and non-IP groups. There were no statistically significant differences between the 2 groups in regards to the thyroid lobe dimensions and volumes. The univariate analysis showed that the IP group was associated with a higher occurrence of LN presence compared to the non-IP group (21% vs. 14%, P=0.023).
Full table
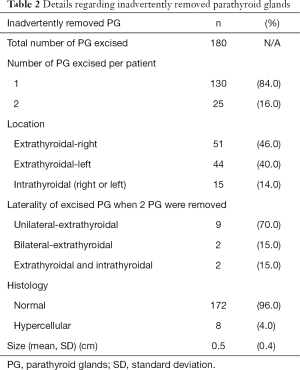
In Table 2, the characteristics of the 180 PG that were inadvertently removed are presented in detail. The percentage of intrathyroidal PG in females was 14% (13/95) and for males was 20% (2/10).
Full table
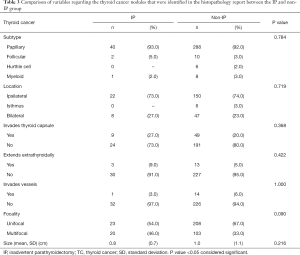
There were no statistically significant differences between the 2 groups (IP and non-IP groups) in regards to the TC-related variables (size, subtype, location, focality and pathological characteristics) (Table 3).
Full table
A logistic regression was performed to ascertain the effects of gender, weight of thyroid gland, gender, thyroid activity pre-operatively, type of operation and the presence of LN, on the likelihood that IP occurred. The logistic regression model was statistically significant and explained the 43% of cases. As seen in Table 4, female gender and the absence of LN in pathology were associated with less likelihood in developing IP than males and patients with presence of LN (P=0.051 and 0.014 respectively). Furthermore, it seems that IP is 2.14 and 2.28 times more likely to occur in TT and NTT respectively, when compared to the combination of TL/NTL (P=0.047 and 0.048 respectively).
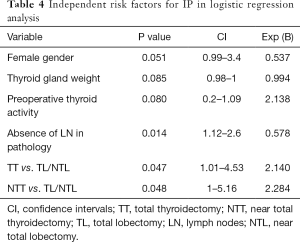
Full table
Discussion
IP is one of the few complications in surgery that continues to be present even in the hands of very experienced surgeons. We have determined the IP rate in a large single centre cohort and investigated the risk factors that are associated with it. Our study has shown that the presence of LN, female gender and the type of operation are significant risk factors for IP, as demonstrated in the logistic regression analysis.
The IP rate in our large cohort of patients was 11.3% which falls within the range of IP rates published previously (3.7–28.0%) (5,8-23). Our study is the first study, to our knowledge, to investigate in depth the effect of the presence of LN to the incidence of IP. We have shown that the presence of benign LN is correlated with a higher incidence of IP. It is noteworthy that the mean sizes of the inadvertently removed PG and LN in our study are very similar (0.5 and 0.4 cm respectively). It seems plausible that the presence of LN hinders the identification of the PG and their safe preservation leading to a higher rate of IP. We did not include in our study cases with central neck dissections as tit would be a confounding factor for assessing the LN status of TT/NTT patients.
In our department the procedure of choice is TT wherever possible while NTT on one side or bilaterally (leaving <3 g of thyroid tissue) is performed whenever the anatomy of the thyroid gland poses a risk to the safe dissection and preservation of the recurrent laryngeal nerve(s). In our study the combination of TT/NTT (TT on one side and NTT on the other side) demonstrated the lowest rates of IP compared to the other two type of procedures performed (TT on both sides and NTT on both sides).
In our study age at date of operation didn’t influence the IP rate while 2 studies have found that younger age were a risk factor for IP (20,24). Younger patients are potentially more likely to undergo operations for malignancy and the 2 studies from Sorgato et al. and Sippel et al. included patients that underwent completion thyroidectomies, lobectomies and LN dissections.
We have demonstrated in both the univariate and multivariate analysis, in this study that females have a lower risk of IP compared to males (P=0.051). The correlation of female gender with a higher incidence of IP has been previously reported (14,25). On the contrary, Manouras et al. and Sakorafas et al. have reported a lower incidence of IP in males and 2 other studies found that sex did not affect the IP rate (9,23,24,26). Females in our study had less intrathyroidal inadvertently removed PG compared to males (14% vs. 20%). Future studies could assess covariates such as body mass index to further investigate these differences.
The evidence in the literature regarding the influence of the final histopathology on the incidence of IP is yet unclear. We found 3 studies supporting a higher incidence of IP in patients with malignancy, 3 other studies did not report such a correlation while the study from Gourgiotis et al. found that thyroid malignancy was associated with a decreased incidence of IP (P=0.047) (9,10,13,14,19,20,24,26). Our study, by excluding cases with central neck dissection, has demonstrated that there is no significant effect of the thyroid pathology on the IP rate when a TT or NTT is performed for a benign disease or a thyroid malignancy (that does not require formal LN clearance).
In our study in the univariate analysis, but not in the multivariate analysis, a smaller weight of the thyroid gland predicted a higher rate of IP (P=0.023). It is interesting that Sippel et al. have also found no correlation between size of the gland and IP while 3 other studies have reported the opposite (14,20,24,25). A small sized thyroid gland may make its handling (rotating the lobe medially to expose the posterior surface where the superior PG normally are found) more challenging from a technical point of view, hence increasing the risk of an IP.
Two previous studies have identified Hashimoto’s thyroiditis as a significant risk factor (one study with 7 patients and the other one with 22 patients with thyroiditis in total) while the study from Sakorafas et al. (50 patients with thyroiditis) did not find any significant correlation between thyroiditis and IP (9,26,27). In our study with 386 patients with thyroiditis, documented in the histopathology report, there was no significant correlation with IP.
In our case series we had 15/1373 (1.1%) patients with an intrathyroidal PG that corresponded to 13.6% (15/180) of all inadvertently removed PGs. The respective numbers reported in the literature are 1.2–4.8% and 16–68.8% (9,17,20,23,27,28). There is considerable deviation between institutions as to what is considered a true PG which may explain why there is such a big range of reported intrathyroidal PGs in the literature. The inadvertent removal of a true intrathyroidal PG is an unavoidable complication that highlights the significance of preserving as many PG as possible from the remaining ones in order to avoid post-operative hypocalcaemia.
The impact of IP on post-operative hypocalcaemia has been well documented in the literature. Most studies have reported that IP doesn’t significantly affect post-operative serum calcium levels (1,4,5,11,12,14-16,19,21,22). Only a small number of studies have positively correlated the occurrence of IP with a higher incidence of hypocalcaemia (8,20,24,27). The reason behind this conflicting evidence probably lies in the differences in the inclusion criteria, hypocalcaemia nomenclature used and local protocols for assessing serum calcium and PTH between different centres. A recent meta-analysis from Edafe et al. of predictors of post-thyroidectomy hypocalcaemia identified Graves' disease and heavier thyroid glands as independent predictors of permanent hypocalcaemia in multivariable analysis, while IP was associated with transient hypocalcaemia [odds ratio (OR) 1.90, 95% CI 1.31–2.74] and female sex (OR 2.28, 95% CI 1.53–3.40) (29).
This study is limited by the well-known biases and limitations of its retrospective nature. We have tried to create a homogeneous cohort of patients by excluding operations other than TT and NTT (hemithyroidectomies, sternotomy, neck dissections). The strengths of the study include its large size, the consistency in performing the operations by a limited number of surgeons and the short time period in which these operations were performed. More research is needed to assess the true impact of the removal of one or more PGs in the postoperative hypocalcaemia and hypoparathyroidism rates.
In conclusion, we present the largest single-centre case series on this topic, to our knowledge. The presence of LN, female gender and the type of operation are positively correlated to the IP rates after a TT, NTT or TT/NTT. These factors could be an early warning sign for the operating surgeon to consider early calcium supplementation if the PG have not been convincingly identified during the operation and found to be intact.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study has been approved by the institutional review board (IRB) of our institution and it conforms to the provisions of in accordance with the Helsinki Declaration as revised in 2013 (No. 2009/23).
References
- Agarwal G, Aggarwal V. Is total thyroidectomy the surgical procedure of choice for benign multinodular goiter? An evidence-based review. World J Surg 2008;32:1313-24. [Crossref] [PubMed]
- Prim MP, de Diego JI, Hardisson D, et al. Factors related to nerve injury and hypocalcemia in thyroid gland surgery. Otolaryngol Head Neck Surg 2001;124:111-4. [Crossref] [PubMed]
- Bergamaschi R, Becouarn G, Ronceray J, et al. Morbidity of thyroid surgery. Am J Surg 1998;176:71-5. [Crossref] [PubMed]
- Shaha AR, Jaffe BM. Parathyroid preservation during thyroid surgery. Am J Otolaryngol 1998;19:113-7. [Crossref] [PubMed]
- Sasson AR, Pingpank JF Jr, Wetherington RW, et al. Incidental parathyroidectomy during thyroid surgery does not cause transient symptomatic hypocalcemia. Arch Otolaryngol Head Neck Surg 2001;127:304-8. [Crossref] [PubMed]
- Surgeons TBAoEaT. Fourth National Audit Report. United Kingdom 2012.
- Reeve T, Thompson NW. Complications of thyroid surgery: how to avoid them, how to manage them, and observations on their possible effect on the whole patient. World J Surg 2000;24:971-5. [Crossref] [PubMed]
- Sitges-Serra A, Gallego-Otaegui L, Suarez S, et al. Inadvertent parathyroidectomy during total thyroidectomy and central neck dissection for papillary thyroid carcinoma. Surgery 2017;161:712-9. [Crossref] [PubMed]
- Sakorafas GH, Stafyla V, Bramis C, et al. Incidental parathyroidectomy during thyroid surgery: an underappreciated complication of thyroidectomy. World J Surg 2005;29:1539-43. [Crossref] [PubMed]
- Gourgiotis S, Moustafellos P, Dimopoulos N, et al. Inadvertent parathyroidectomy during thyroid surgery: the incidence of a complication of thyroidectomy. Langenbecks Arch Surg 2006;391:557-60. [Crossref] [PubMed]
- Zhou HY, He JC, McHenry CR. Inadvertent parathyroidectomy: incidence, risk factors, and outcomes. J Surg Res 2016;205:70-5. [Crossref] [PubMed]
- Rix TE, Sinha P. Inadvertent parathyroid excision during thyroid surgery. Surgeon 2006;4:339-42. [Crossref] [PubMed]
- Hone RW, Tikka T, Kaleva AI, et al. Analysis of the incidence and factors predictive of inadvertent parathyroidectomy during thyroid surgery. J Laryngol Otol 2016;130:669-73. [Crossref] [PubMed]
- Rajinikanth J, Paul MJ, Abraham DT, et al. Surgical audit of inadvertent parathyroidectomy during total thyroidectomy: incidence, risk factors, and outcome. Medscape J Med 2009;11:29. [PubMed]
- Abboud B, Sleilaty G, Braidy C, et al. CAreful examination of thyroid specimen intraoperatively to reduce incidence of inadvertent parathyroidectomy during thyroid surgery. Arch Otolaryngol Head Neck Surg 2007;133:1105-10. [Crossref] [PubMed]
- Lee NJ, Blakey JD, Bhuta S, et al. Unintentional parathyroidectomy during thyroidectomy. Laryngoscope 1999;109:1238-40. [Crossref] [PubMed]
- Erbil Y, Barbaros U, Ozbey N, et al. Risk factors of incidental parathyroidectomy after thyroidectomy for benign thyroid disorders. Int J Surg 2009;7:58-61. [Crossref] [PubMed]
- Sheahan P, Mehanna R, Basheeth N, et al. Is systematic identification of all four parathyroid glands necessary during total thyroidectomy?: a prospective study. Laryngoscope 2013;123:2324-8. [Crossref] [PubMed]
- Lin DT, Patel SG, Shaha AR, et al. Incidence of inadvertent parathyroid removal during thyroidectomy. Laryngoscope 2002;112:608-11. [Crossref] [PubMed]
- Sorgato N, Pennelli G, Boschin IM, et al. Can we avoid inadvertent parathyroidectomy during thyroid surgery? In Vivo 2009;23:433-9. [PubMed]
- Page C, Strunski V. Parathyroid risk in total thyroidectomy for bilateral, benign, multinodular goitre: report of 351 surgical cases. J Laryngol Otol 2007;121:237-41. [Crossref] [PubMed]
- Irkorucu O, Tascilar O, Cakmak GK, et al. Inadvertent parathyroidectomy and temporary hypocalcemia: an adverse natural outcome or a true complication during thyroidectomy? Endocr Regul 2007;41:143-8. [PubMed]
- Manouras A, Markogiannakis H, Lagoudianakis E, et al. Unintentional parathyroidectomy during total thyroidectomy. Head Neck 2008;30:497-502. [Crossref] [PubMed]
- Sippel RS, Ozgul O, Hartig GK, et al. Risks and consequences of incidental parathyroidectomy during thyroid resection. ANZ J Surg 2007;77:33-6. [Crossref] [PubMed]
- Zhou HY, He JC, McHenry CR. Inadvertent parathyroidectomy: incidence, risk factors, and outcomes. J Surg Res 2016;205:70-5. [Crossref] [PubMed]
- Abboud B, Sleilaty G, Braidy C, et al. Careful examination of thyroid specimen intraoperatively to reduce incidence of inadvertent parathyroidectomy during thyroid surgery. Arch Otolaryngol Head Neck Surg 2007;133:1105-10. [Crossref] [PubMed]
- Khairy GA, Al-Saif A. Incidental parathyroidectomy during thyroid resection: incidence, risk factors, and outcome. Ann Saudi Med 2011;31:274-8. [Crossref] [PubMed]
- Del Rio P, De Simone B, Viani L, et al. Unintentional parathyroidectomy and postoperative hypocalcaemia. Conventional thyroidectomy versus miniinvasive thyroidectomy. Ann Ital Chir 2014;85:470-3. [PubMed]
- Edafe O, Antakia R, Laskar N, et al. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014;101:307-20. [Crossref] [PubMed]