To identify or not to identify parathyroid glands during total thyroidectomy
Introduction
Total thyroidectomy is a common surgical procedure and is often the treatment of choice for a number of benign and malignant conditions. However, despite being a relatively safe procedure in experienced hands, hypoparathyroidism remains one of the most common complications (1,2). Patients suffering from postoperative hypoparathyroidism generally present with a low postoperative serum calcium (Ca), a high phosphate and a low parathyroid hormone (PTH) level. Symptomatic hypocalcemia (such as numbness, muscle spasms, confusion) may occur, although it is getting less common because of the improved postoperative Ca management (3). Regarding the etiology of postoperative hypoparathyroidism, it is generally thought that it is due to a transient reduction in blood supply of in situ parathyroid glands (PGs), which results in a transient reduction of parathyroid function (or hormone secretion). Although inadvertent removal of PGs (i.e., incidental parathyroidectomy) may contribute to hypoparathyroidism, it does not happen often. As a result, provided the PG is not entirely stripped of its blood supply, normalization of parathyroid function is expected for the majority of patients. Nevertheless, this recovery phase may take several weeks to months (i.e., protracted hypoparathyroidism) (2). After normalization, patients are usually able to stop their oral Ca ± calcitriol supplements. However, a small proportion (<5%) may not have their parathyroid function normalized and they are considered as having persistent or permanent hypoparathyroidism. The reported rates of temporary and permanent hypoparathyroidism in the literature vary widely between 19% to 38% and 0% to 3% respectively, depending on the definition of hypoparathyroidism (4), casemix and type of thyroid resections.
Although postoperative hypoparathyroidism can often be managed by taking regular oral Ca ± vitamin D supplements, some patients may experience persistent hypocalcemic symptoms leading to impaired daily living and quality of life. This situation also poses challenges to clinicians and increases health costs (5-7). Long term morbidities such as renal failure, basal ganglia calcifications, neuropsychiatric derangements and infections have been reported as a result of supplementation over a prolonged period (8,9).
Risk factors leading to postoperative hypoparathyroidism
The extent of resection and surgical technique are factors known to influence the subsequent risk of postoperative hypoparathyroidism (10). For instance, patients who undergo a bilateral thyroidectomy are at greater risk of hypoparathyroidism than those who undergo a unilateral thyroidectomy (10,11). Similarly, those who undergo peripheral ligation of the inferior thyroid artery at the thyroid capsule are more prone to develop hypoparathyroidism than those who undergo sub-capsular dissection (10). Parathyroid auto-transplantation, inadvertent excision of PGs and presence of PGs in the thyroid specimen have also been shown to increase the risk of post-operative hypoparathyroidism (1,11-17). This is because they all lower the number of in situ PGs and therefore, the residual parathyroid function is immediately compromised. Concomitant central neck dissection has also been found to be an independent risk factor, presumably because this procedure increases risk of incidental parathyroidectomy (15,16). Surgeon’s experience (12) and hospital operative volume (11) have also been shown to be important in post-operative hypoparathyroidism. After operation, clinical symptoms and signs such as numbness and tetany, as well as serum Ca level should be monitored. Some suggested that an early postoperative decrease in serum iPTH concentrations (8,10), combining with serum Ca concentration may predict hypoparathyroidism and guide the administration of Ca or vitamin D supplements (18,19). Post-operative hypoparathyroidism results from a reduction of functioning parathyroid parenchyma, which could be secondary to intraoperative damages caused by mechanical or thermal trauma, gland devascularization, obstruction of venous outflow, and inadvertent parathyroid excision (14,15,17). Therefore, the question has been whether there are strategies or approaches a surgeon could follow intra-operatively to minimize PG injuries and hypoparathyroidism. One approach has been to see if there is a relationship between the number of PGs identified intra-operatively and post-operative hypoparathyroidism. To date, there are two schools of thought with some surgeons advocating routine identification of all PGs (i.e., the operating surgeon spending time in visualizing all or as many PGs as possible at surgery) while others advocating the opposite and would spend little to no time in visualizing PGs at surgery (i.e., selective identification).
Routine versus selective identification
The term “routine identification” refers to a strategy where the surgeon would try his or her best to identify each and every PG in its orthotopic or non-orthotopic position (20-23). This has traditionally been the approach accepted by many surgeons. However, the pitfall with this strategy is that not all PGs could be found in their orthotopic or usual positions. For example, some superior PGs are located at the superior pole of the posterior thyroid gland near the cricothyroid junction and some inferior PGs may be located far away from the neck in the thymus and mediastinum (24,25). Therefore, even with the best intention, it may not be always possible to identify each and every PG at the time of total thyroidectomy. The other pitfall is that this strategy may lead to inadvertent damage to the nearby blood supply and therefore, may devitalize PGs leading hypoparathyroidism. Also, this strategy may unnecessarily prolong the operation.
On the other hand, some surgeons advocate a selective approach where the PGs are encountered rather than being actively searched for during surgery. This approach might be more time-saving as the surgeon does not need to search all sites and because of the less extensive dissection, the chance of inadvertently jeopardizing the blood supply to PGs would be less. Nevertheless, in either approaches, when parathyroid blood supply is jeopardized, expeditious autografting of the affected PG should be performed, although the benefit of autografting has also been questioned (26,27).
Supporting evidence for either approach
Given that the issue of whether to routinely identify or not identify PGs during total thyroidectomy has remained controversial, the present review aimed to look into the current literature for supporting evidence in either approach. To improve the clarity of the evidence, studies were divided into those reporting temporary (<6 months) and those reporting persistent/permanent (≥6 months) after operation. For studies reporting both, they were included in both tables.
The evidence for routine identification
Table 1 lists the studies which support the routine identification approach.
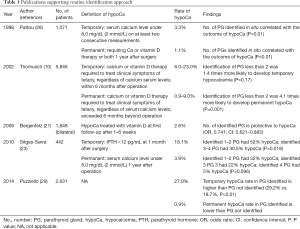
Full table
Temporary hypocalcemia
Bergenfelz et al. reported a multicenter retrospective audit with 3,660 patients, involving both benign and malignant thyroid diseases and various types of thyroid operations. Among these, 1,648 patients received bilateral thyroidectomy. It was found that at 6-week post-op, 10% and 8% of patients were on vitamin D and Ca supplements, respectively; and the number of identified PGs intra-operatively was protective to hypocalcemia [odds ratio (OR), 0.74; confidence interval (CI): 0.621–0.883]. Association with hypocalcemia at 6-month post-op was not analyzed since missing data was high (4.5%) (21).
Siteges-Serra et al. reported the outcome of post-thyroidectomy hypocalcemia in 422 patients retrospectively, and found that 50.2% developed hypocalcemia post-operatively, which was defined as Ca less than 2.0 mmol/L (23). At one month after operation the rate of hypoparathyroidism was 18.1%; and within the group of patients who had post-operative hypocalcemia, identifying 3 or 4 PGs had less hypocalcemia than identifying 1 or 2 PGs (30.5% vs. 52.0%, P=0.018). However, one fifth of their patients underwent central neck dissection for thyroid cancer and the results were not adjusted for confounding factors. The rate of hypocalcemia at 1 year after operation was not associated with the number of PGs identified.
Permanent hypocalcemia
A German retrospective multivariate analysis by Thomusch et al. involving 5,846 patients underwent bilateral thyroidectomy showed that besides extent of thyroid operations and surgical technique, number of identified PGs less than 2 increased post-operative permanent hypocalcemia (OR, 4.1; P=0.001), which was defined as requiring Ca or vitamin D supplements 6 months after operation (10). Removal of a single PG was not associated with post-operative hypoparathyroidism. Similar trend was observed for transient hypocalcemia (less than 6 months) however statistical significance was not reached (OR, 1.7; P=0.700). Together with some other reports which showed that permanent hypoparathyroidism emerged exclusively after less than 3 PGs had been identified intra-operatively (22,30), it was suggested that at least 2 PGs should be identified and preserved in situ during operation (10).
This suggestion was also supported by Pattou et al. (28), who prospectively evaluated the incidence and predictive factors of hypocalcemia in 1,071 consecutive patients. It was found that number of PGs identified in situ during surgery was correlated with the outcome of hypocalcemia (P=0.010). Patients carried a high risk for permanent hypocalcemia (Ca <2 mmol/L and symptomatic 1 year after operation) if fewer than 3 PGs were identified in situ during surgery. When 3 or more PGs identified and preserved during surgery, spontaneous recovery was observed in all patients except one (28).
Similar findings were reported in a recent multi-centre prospective study by Puzziello et al. involving 2,631 patients, showing that identifying PGs was important to prevent permanent hypocalcemia. Interestingly, the percentage of developing transient hypocalcemia in patients with identification of PGs intra-operatively was higher than in patients with PGs not identified (29.2% vs. 18.7%, P<0.010). This suggested that identifying PGs during operation may have a protective role in hypocalcemia in long-term, though in expense of a higher risk of hypocalcemia in short term (29).
The evidence for selective identification
In contrast, some recent studies showed that patients with a greater number of PGs identified intra-operatively might be at greater risk of hypoparathyroidism. Some suggest that identification of PGs is not equivalent to preserving them; instead, may increase direct trauma or disrupt blood supply during surgical manipulation (29,31). Therefore, surgeons start to advocate “selective approach” while PGs are only sought in their orthotopic locations with no attempt to identify them elsewhere. It was hypothesized that with the adoption of extra-capsular dissection, not seeing a PG usually implies that it is being covered by vascularized tissue and therefore had a better chance of being in situ (31). Of course, this assumption is only valid in high quality extra-capsular dissection and experienced hands. Table 2 summarizes the evidence in support of selective identification approach.
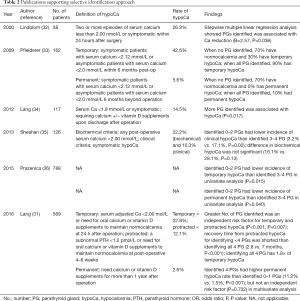
Full table
Temporary hypocalcemia
In 2000, a small Swedish prospective cohort by Lindblom et al. with 38 patients undergone total or near total thyroidectomy reported that number PGs identified intra-operatively was associated with Ca reduction within 24 hours after operation via stepwise multiple linear regression (B=2.57, P=0.036) (32). Recently, Lang et al. reported a prospective study with 117 patients evaluating predictive factors of hypocalcemia after thyroidectomy and found that the more PGs identified intra-operatively, the more post-operative transient hypocalcemia (P=0.017) (34). Later the same group conducted another prospective study with 569 patients to specifically look into the relationship between number of PGs identified intra-operatively with post-operative hypocalcemia, and confirmed that greater number of PGs identified was an independent risk factor for temporary (within 24 hours) and protracted (4–6 weeks after operation) hypocalcemia (P<0.001, P=0.007, respectively). Recovery time from protracted hypocalcemia for identifying <4 PGs was significantly shorter than identifying all 4 PGs (2.8 vs. 7 months, P<0.001). Chance of having 4 glands in situ decreased with greater number of PGs identified and identifying all 4 PGs has 1.8 times risk of temporary hypocalcemia (31).
Similar results were found when comparing patients with 0–2 to 3–4 PGs seen intra-operatively. In a prospective study by Sheahan et al. with 126 patients underwent total thyroidectomy, 0–2 PGs identified has lower incidence of clinical hypocalcemia than 3–4 PGs found (3.2% vs. 17.1%, P=0.02). No difference was observed in inadvertent parathyroidectomy between the two groups, which might suggest routine identification of PGs may not be helpful in preventing inadvertent parathyroidectomy (35). In a retrospective study by Prazenica et al. with 788 patients, higher temporary hypocalcemia (P=0.015) was found in 3–4 PGs identified group (36). In the study of Puzziello et al. (29), although it suggested that number of identified PGs may increase permanent hypocalcemia, it was associated with more transient hypocalcemia.
Permanent hypocalcemia
Pflederer et al. reported a prospective cohort involving 162 patients who underwent total or completion thyroidectomy (33). Association test between number of PGs identified and the development of hypocalcemia produced a significant result in post-operative 6 months (Chi =11.4, P=0.022). When no PGs were seen, 70% had normo-calcemia and 30% has temporary hypocalcemia. For those with all PGs identified, 90% developed temporary hypocalcemia and 10% with permanent hypocalcemia, which required exogenous Ca and vitamin D beyond 6 months after operation (33).
Similarly, a recently published prospective study by Lang et al. with 569 patients who underwent total thyroidectomy for benign disease reported that permanent hypocalcemia, which was defined as requiring exogenous Ca or vitamin D one year after operation, was significantly higher in patient with 4 PGs identified than in patients with 0–1 PGs identified (11.2% vs. 1.5%, P<0.001). Although the number of PGs identified was not an independent risk factor (P=0.702) in multivariate analysis, this might be due to small number of permanent hypocalcemia in the study (n=15, 2.6%) (31). Similarly, Prazenica et al. also found that identifying 4 PGs intra-operatively had higher permanent hypocalcemia rate than identifying 0–1 PGs in univariate analysis (P=0.040) (36).
Shortcomings with the literature
It is worth noting that majority of the studies were retrospective in design, for instance, multi-institutional observational studies and audits, thus only the association but not the causative relationship can be determined. Moreover, the low incidence of permanent hypoparathyroidism, which was reported to be 0–3.6% (1), leads to lack of statistical significance in the association test between number of identified PGs and permanent hypocalcemia. Although multi-institutional studies provide large sample size, they involved a heterogeneous population with different inclusion and exclusion criteria, various extents of thyroid resections (total or subtotal thyroidectomy, bilateral lobectomy, completion thyroidectomy, central neck dissection, etc.), different definition of hypoparathyroidism, drug prescription and follow-up protocol. These may miss out certain confounding factors that give rise to postsurgical hypoparathyroidism (1,17). On the other hand, single-institutional studies provide clearer definition and better quality data. However, they were frequently under power due to small sample size and the results may not be applicable in other institutions due to different volume of operations and surgeon’s experience.
A systemic review and meta-analysis focusing on reducing post-thyroidectomy hypocalcemia found that no measures, including intra-operative PG identification, were significantly associated with reduction of hypocalcemia, given the majority of trials were of low quality due to a lack of blinding and also wide variability in study design and definitions (37). So far there is no meta-analysis specifically summarizes the relationship of intra-operative identifications of PGs with post-operative hypoparathyroidism; understandingly it would be difficult because of the very heterogeneous studies. Moreover, an inevitable limitation for all studies is that PGs were “identified” in situ solely based on operative surgeon’s assessment without any histological proof; understandingly biopsy of the identified PG may actually inflict more damage and thus lead to more iatrogenic hypoparathyroidism. In addition, the current available studies only focus on the relationship between number of identified PGs and hypocalcemia, which may not truly reflect on the comparison between routine and selective identification approach. A lot of time PGs were encountered during dissection without surgeon’s intention to look for them. Thus, a high number of PGs identified is not necessarily equivalent to adopting a routine identification approach. It is also difficult to have carried out prospective study or randomized controlled trial to compare these two approaches, since a large number of patients would be required to ensure adequate power.
Relationship between number of identified PGs and parathyroid function
Despite the conflicting data in the literature, one thing is clear: to reduce the risk of postoperative hypoparathyroidism, the ability of leaving as many “vascularized” PGs in situ is very important. In a prospective study of 657 patients who underwent first-time total thyroidectomy, the prevalence of hypocalcemia and of protracted and permanent hypoparathyroidism were inversely related to the PGs remaining in situ score (i.e., 4 minus “auto-transplanted PGs” minus “PGs in specimen”). By logistic regression, this score turned out to be one of the most significant variables influencing acute and chronic parathyroid failures. On the other hand, the number of PGs identified intraoperatively did not appear to have any impact on transient, protracted or permanent hypocalcemia (38).
Similarly, in a retrospective review of 454 patients who underwent total thyroidectomy for papillary thyroid carcinoma, the number of PGs preserved was obtained by subtracting the number of PGs in a given specimen from 4. It was found that the incidence of transient hypoparathyroidism increased when there were three or fewer preserved PGs than when all four PGs were preserved (P=0.004), but did not affect permanent hypoparathyroidism. During total thyroidectomy, preserving at least one PG with an intact blood supply appeared to be sufficient to prevent permanent hypoparathyroidism when auto-transplant was not performed (39). Lang et al. also showed that the number of PG in situ was inversely proportional to post-thyroidectomy temporary, protracted and permanent hypoparathyroidism (31). In a study about multi-factorial scoring system to predict post thyroidectomy hypocalcemia so as to enable safe discharge within 24 hours of surgery with 145 patients, lesser number of PG preserved at surgery was one of the significant predictive factors (P=0.001) (40).
Identifying PG is not a safeguard for protecting its function, because how we adequately assess the viability of the PG after finding it out based on macroscopic appearance is questionable due to subjective assessment and inter-observant variation. Moreover, function of discolored glands was not necessarily impaired and may recover within a short time after surgery (41). On the other hand, absence of discoloration was not a reliable way to suggest intact PG blood supply (42). Selective auto-transplantation was advocated for de-vascularized or inadvertent removed PGs. However, for PGs that were identified in situ, an intact vascular pedicle seemed cannot guarantee adequate function of PGs, because protracted and permanent hypoparathyroidism were also seen in patients with PGs that seemingly had adequate blood supply (43). Occasionally a PG can look normal even though its vascular pedicle seemed to be unsafe, and some surgeons evaluated blood supply for controversial PG by making a small incision in it with a cold knife (44). Yet it was also found that surgeons differ substantially in the ability of predicting risk of hypoparathyroidism during thyroid surgery (45). Thus, an objective and less invasive assessment is warranted.
Assessing viability of in situ PGs
So now the question continued on how we could assess parathyroid viability in real-time, and whether it is possible to identify and preserve PGs in situ without trying to dissect and expose them out, limiting the risk of vascular injury. Studies have been going on for locating and predicting function of PGs with indocyanine green (ICG) fluorescent imaging in both thyroid and parathyroid surgery (46-52). ICG is a water-soluble molecule that binds to plasma protein and confined to intravascular compartment. It will emit fluorescent light when being excited by near-infrared light (NIR); thus, the combination of ICG and NIR may provide real-time assessment on tissue perfusion within a focused area reflected by the fluorescent light intensity. ICG fluorescent imaging potentially helps locate PGs and thus prevent inadvertent parathyroidectomy or damaging them (48). Moreover, ICG angiography may provide quantitative evaluation of in situ PG perfusion after total thyroidectomy, and could be a good predictor for post-operative hypoparathyroidism (46,47,50,51). Although currently these reported studies were mainly concerning feasibility with a relative small sample size, ICG fluorescent angiography could potentially be a less invasive option for identifying PGs during thyroidectomy and provide more accurate prediction of parathyroid function.
Conclusions
It remains controversial on how the number of PGs identified during total thyroidectomy may affect iatrogenic hypoparathyroidism. More PGs identified during surgery appear to be associated with a greater risk of temporary hypoparathyroidism. Regarding to permanent hypoparathyroidism, conclusion remains unclear because of the low incidence and heterogeneity of studies. Moreover, one should realize that studying the association between numbers of PGs identified intra-operatively and post-thyroidectomy hypoparathyroidism did not directly address on the issue of whether or not the surgeon should actively seek all PGs regardless of their location. To better answer this question, to identify or not identify the PGs during total thyroidectomy, a prospective comparative study or even a randomized control trial might be required, although this would be difficult due to requirement of large sample size. In order to prevent iatrogenic hypocalcemia, the traditional teaching of leaving at least one vascularized pedicle attached to an in situ PG still remains true. With the new advancement in ICG fluorescent angiographic technology, whether to identify or not identify PGs also depends on the method to localize and assess its function in a non-invasive manner.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Edafe O, Antakia R, Laskar N, et al. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014;101:307-20. [Crossref] [PubMed]
- Lorente-Poch L, Sancho JJ, Munoz-Nova JL, et al. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg 2015;4:82-90. [PubMed]
- Kakava K, Tournis S, Papadakis G, et al. Postsurgical Hypoparathyroidism: A Systematic Review. In Vivo 2016;30:171-9. [PubMed]
- Mehanna HM, Jain A, Randeva H, et al. Postoperative hypocalcemia--the difference a definition makes. Head Neck 2010;32:279-83. [PubMed]
- Cho NL, Moalem J, Chen L, et al. Surgeons and patients disagree on the potential consequences from hypoparathyroidism. Endocr Pract 2014;20:427-46. [Crossref] [PubMed]
- Hadker N, Egan J, Sanders J, et al. Understanding the burden of illness associated with hypoparathyroidism reported among patients in the PARADOX study. Endocr Pract 2014;20:671-9. [Crossref] [PubMed]
- Wang TS, Cheung K, Roman SA, et al. To supplement or not to supplement: a cost-utility analysis of calcium and vitamin D repletion in patients after thyroidectomy. Ann Surg Oncol 2011;18:1293-9. [Crossref] [PubMed]
- Mitchell DM, Regan S, Cooley MR, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab 2012;97:4507-14. [Crossref] [PubMed]
- Underbjerg L, Sikjaer T, Mosekilde L, et al. Postsurgical hypoparathyroidism--risk of fractures, psychiatric diseases, cancer, cataract, and infections. J Bone Miner Res 2014;29:2504-10. [Crossref] [PubMed]
- Thomusch O, Machens A, Sekulla C, et al. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 2003;133:180-5. [Crossref] [PubMed]
- Abboud B, Sargi Z, Akkam M, et al. Risk factors for postthyroidectomy hypocalcemia. J Am Coll Surg 2002;195:456-61. [Crossref] [PubMed]
- Paek SH, Lee YM, Min SY, et al. Risk factors of hypoparathyroidism following total thyroidectomy for thyroid cancer. World J Surg 2013;37:94-101. [Crossref] [PubMed]
- McLeod IK, Arciero C, Noordzij JP, et al. The use of rapid parathyroid hormone assay in predicting postoperative hypocalcemia after total or completion thyroidectomy. Thyroid 2006;16:259-65. [Crossref] [PubMed]
- Sitges-Serra A, Gallego-Otaegui L, Suarez S, et al. Inadvertent parathyroidectomy during total thyroidectomy and central neck dissection for papillary thyroid carcinoma. Surgery 2017;161:712-9. [Crossref] [PubMed]
- Zhou HY, He JC, McHenry CR. Inadvertent parathyroidectomy: incidence, risk factors, and outcomes. J Surg Res 2016;205:70-5. [Crossref] [PubMed]
- Chisholm EJ, Kulinskaya E, Tolley NS. Systematic review and meta-analysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone. Laryngoscope 2009;119:1135-9. [Crossref] [PubMed]
- Cho JN, Park WS, Min SY. Predictors and risk factors of hypoparathyroidism after total thyroidectomy. Int J Surg 2016;34:47-52. [Crossref] [PubMed]
- Vanderlei FA, Vieira JG, Hojaij FC, et al. Parathyroid hormone: an early predictor of symptomatic hypocalcemia after total thyroidectomy. Arq Bras Endocrinol Metabol 2012;56:168-72. [Crossref] [PubMed]
- Lecerf P, Orry D, Perrodeau E, et al. Parathyroid hormone decline 4 hours after total thyroidectomy accurately predicts hypocalcemia. Surgery 2012;152:863-8. [Crossref] [PubMed]
- Dabholkar JP, Chirmade S, Chhapola S. Safe thyroidectomy: Our view point. Indian J Otolaryngol Head Neck Surg 2006;58:222-4. [PubMed]
- Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg 2008;393:667-73. [Crossref] [PubMed]
- Thomusch O, Machens A, Sekulla C, et al. Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: prospective multicenter study in Germany. World J Surg 2000;24:1335-41. [Crossref] [PubMed]
- Sitges-Serra A, Ruiz S, Girvent M, et al. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg 2010;97:1687-95. [Crossref] [PubMed]
- Fancy T, Gallagher D 3rd, Hornig JD. Surgical anatomy of the thyroid and parathyroid glands. Otolaryngol Clin North Am 2010;43:221-7. vii. [Crossref] [PubMed]
- Melo C, Pinheiro S, Carvalho L, et al. Identification of parathyroid glands: anatomical study and surgical implications. Surg Radiol Anat 2015;37:161-5. [Crossref] [PubMed]
- Lorente-Poch L, Sancho J, Munoz JL, et al. Failure of fragmented parathyroid gland autotransplantation to prevent permanent hypoparathyroidism after total thyroidectomy. Langenbecks Arch Surg 2017;402:281-7. [Crossref] [PubMed]
- Tartaglia F, Blasi S, Giuliani A, et al. Parathyroid autotransplantation during total thyroidectomy. Results of a retrospective study. Int J Surg 2016;28 Suppl 1:S79-83. [Crossref] [PubMed]
- Pattou F, Combemale F, Fabre S, et al. Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg 1998;22:718-24. [Crossref] [PubMed]
- Puzziello A, Rosato L, Innaro N, et al. Hypocalcemia following thyroid surgery: incidence and risk factors. A longitudinal multicenter study comprising 2,631 patients. Endocrine 2014;47:537-42. [Crossref] [PubMed]
- Olson JA Jr, DeBenedetti MK, Baumann DS, et al. Parathyroid autotransplantation during thyroidectomy. Results of long-term follow-up. Ann Surg 1996;223:472-8; discussion 478-80. [Crossref] [PubMed]
- Lang BH, Chan DT, Chow FC. Visualizing fewer parathyroid glands may be associated with lower hypoparathyroidism following total thyroidectomy. Langenbecks Arch Surg 2016;401:231-8. [Crossref] [PubMed]
- Lindblom P, Westerdahl J, Bergenfelz A. Low parathyroid hormone levels after thyroid surgery: a feasible predictor of hypocalcemia. Surgery 2002;131:515-20. [Crossref] [PubMed]
- Pfleiderer AG, Ahmad N, Draper MR, et al. The timing of calcium measurements in helping to predict temporary and permanent hypocalcaemia in patients having completion and total thyroidectomies. Ann R Coll Surg Engl 2009;91:140-6. [Crossref] [PubMed]
- Lang BH, Yih PC, Ng KK. A prospective evaluation of quick intraoperative parathyroid hormone assay at the time of skin closure in predicting clinically relevant hypocalcemia after thyroidectomy. World J Surg 2012;36:1300-6. [Crossref] [PubMed]
- Sheahan P, Mehanna R, Basheeth N, et al. Is systematic identification of all four parathyroid glands necessary during total thyroidectomy?: a prospective study. Laryngoscope 2013;123:2324-8. [Crossref] [PubMed]
- Praženica P, O'Keeffe L, Holy R. Dissection and identification of parathyroid glands during thyroidectomy: association with hypocalcemia. Head Neck 2015;37:393-9. [Crossref] [PubMed]
- Antakia R, Edafe O, Uttley L, et al. Effectiveness of preventative and other surgical measures on hypocalcemia following bilateral thyroid surgery: a systematic review and meta-analysis. Thyroid 2015;25:95-106. [Crossref] [PubMed]
- Lorente-Poch L, Sancho JJ, Ruiz S, et al. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 2015;102:359-67. [Crossref] [PubMed]
- Song CM, Jung JH, Ji YB, et al. Relationship between hypoparathyroidism and the number of parathyroid glands preserved during thyroidectomy. World J Surg Oncol 2014;12:200. [Crossref] [PubMed]
- Pradeep PV, Ramalingam K, Jayashree B. Post total thyroidectomy hypocalcemia: A novel multi-factorial scoring system to enable its prediction to facilitate an early discharge. J Postgrad Med 2013;59:4-8. [Crossref] [PubMed]
- Promberger R, Ott J, Kober F, et al. Intra- and postoperative parathyroid hormone-kinetics do not advocate for autotransplantation of discolored parathyroid glands during thyroidectomy. Thyroid 2010;20:1371-5. [Crossref] [PubMed]
- Kuhel WI, Carew JF. Parathyroid biopsy to facilitate the preservation of functional parathyroid tissue during thyroidectomy. Head Neck 1999;21:442-6. [Crossref] [PubMed]
- Lang BH, Chan DT, Chow FC, et al. The Association of Discolored Parathyroid Glands and Hypoparathyroidism Following Total Thyroidectomy. World J Surg 2016;40:1611-7. [Crossref] [PubMed]
- Ji YB, Song CM, Sung ES, et al. Postoperative Hypoparathyroidism and the Viability of the Parathyroid Glands During Thyroidectomy. Clin Exp Otorhinolaryngol 2017;10:265-71. [Crossref] [PubMed]
- Promberger R, Ott J, Bures C, et al. Can a surgeon predict the risk of postoperative hypoparathyroidism during thyroid surgery? A prospective study on self-assessment by experts. Am J Surg 2014;208:13-20. [Crossref] [PubMed]
- Lang BH, Wong CK, Hung HT, et al. Indocyanine green fluorescence angiography for quantitative evaluation of in situ parathyroid gland perfusion and function after total thyroidectomy. Surgery 2017;161:87-95. [Crossref] [PubMed]
- Lavazza M, Liu X, Wu C, et al. Indocyanine green-enhanced fluorescence for assessing parathyroid perfusion during thyroidectomy. Gland Surg 2016;5:512-21. [Crossref] [PubMed]
- Yu HW, Chung JW, Yi JW, et al. Intraoperative localization of the parathyroid glands with indocyanine green and Firefly(R) technology during BABA robotic thyroidectomy. Surg Endosc 2017;31:3020-7. [Crossref] [PubMed]
- Ladurner R, Sommerey S, Arabi NA, et al. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc 2017;31:3140-5. [Crossref] [PubMed]
- Zaidi N, Bucak E, Yazici P, et al. The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 2016;113:775-8. [Crossref] [PubMed]
- Vidal Fortuny J, Belfontali V, Sadowski SM, et al. Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 2016;103:537-43. [Crossref] [PubMed]
- Sound S, Okoh A, Yigitbas H, et al. Utility of Indocyanine Green Fluorescence Imaging for Intraoperative Localization in Reoperative Parathyroid Surgery. Surg Innov 2015. [Epub ahead of print].


