Natural history of metaplastic squamous cell breast cancer: a case report and literature review on surgical management
Introduction
Metaplastic breast cancer is a rare and heterogeneous entity. It consists of a variety of neoplasms with predominantly non glandular differentiation of spindle cell, squamous, and/or mesenchymal origin. Metaplastic squamous cell breast cancer, in particular, is an extremely rare subtype, accounting for less than 1% of all invasive breast cancers (1). As a result, there is sparse data in literature relating to its presentation and management. We aim to report the natural behaviour of metaplastic squamous cell breast cancer which was picked up on screening mammogram. The surgical management of this rare entity was reviewed.
Case presentation
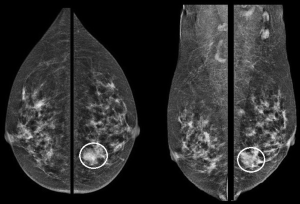
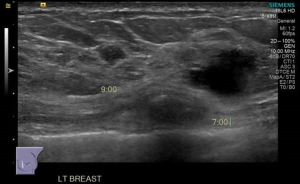
The patient was a 62-year-old asymptomatic lady. Clinical examination was unremarkable. Her screening mammogram revealed a left lower inner quadrant asymmetric density (Figure 1) which corresponded to a suspicious 1.5 cm lesion at 7 o’clock position on ultrasound. The ultrasound also showed another indeterminate 0.5 cm lesion in close proximity to the former lesion (Figure 2) and an indeterminate axillary lymph node.
The patient underwent a core-cut needle biopsy which revealed intermediate grade invasive ductal carcinoma (IDC), not otherwise specified (NOS) admixed with ductal carcinoma in situ (DCIS) of the 7 o’clock lesion and DCIS of the smaller lesion. Estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) were negative, weakly positive and negative, respectively. CK14 (a marker for squamous differentiation) was negative. Biopsy of the left axillary node was negative for malignancy and her staging scans revealed no evidence of distant metastasis.
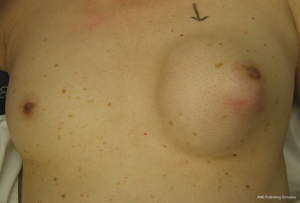
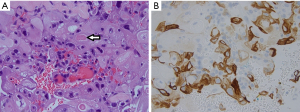
The patient was initially offered breast conservation but she declined further treatment. She returned about 3 months later with a clinically palpable 5 cm left breast 7 o’clock lump (Figure 3) and was now keen for surgery. In view of the large tumour to breast ratio, a left mastectomy with sentinel lymph node biopsy was performed instead. Final histopathology revealed a single 55-mm cystic lesion of grade III IDC, metaplastic subtype with squamous differentiation (Figure 4A) and high grade DCIS. Tumour was triple negative, CK14 was positive (Figure 4B) and sentinel lymph node biopsy was negative.
Postoperatively, the patient recovered well but refused adjuvant chemotherapy and radiotherapy.
Discussion
Metaplastic squamous cell breast cancer is a rare form of cancer and little is known of its presentation and treatment (1). Clinically, these patients had been reported to present with an average palpable breast lump size of more than 4 cm at diagnosis, which is larger than that of breast adenocarcinoma (2). Though metaplastic cancer of high grade squamous subtype has been known to be rapidly growing (3), the extent of rapid growth is not well documented as all the reports relied on the patient’s account and may hence have recall bias. We report the first case of initially asymptomatic metaplastic squamous cell breast cancer, presenting on screening mammogram, which progressed to a large palpable cystic mass in the span of three months because of delayed treatment by the patient, objectively capturing the natural progression of this rare subtype.
Interestingly, on imaging, this patient had an initial multifocal presentation which was not typical of metaplastic squamous cell breast cancer. The smaller lesion, we believe, was most likely an incidental finding of DCIS rather than a true focus of metaplastic squamous cell breast cancer. The unifocal cystic mass on final surgical histology, in our case, correlated with the known metaplastic squamous cancer presentation of a cystic mass on imaging which could occur in over 30–70% of cases (3-5). Mammogram often has no distinctive imaging features from IDC and the use of other breast imaging modalities such as magnetic resonance imaging (MRI) and tomosynthesis are not well reported (6).
Histologically, metaplastic squamous cell cancer can be elusive to diagnose on fine needle aspiration cytology (FNAC) or, as demonstrated in our case, on core-cut needle biopsy (2) which was initially reported as IDC and could possibly be due to tumour heterogeneity and sampling. There is no international consensus on the exact percentage of squamous component needed to classify the tumour as metaplastic squamous cell carcinoma and the values range from >10–50% or more (7). On morphology, they exhibit features of squamous differentiation including keratinisation within the tumour or presence of intercellular bridges between the cells. On immune-staining, the foci of squamous differentiation seen on morphology are positive for CK14/CK5-6/p63/EGFR (8,9). Caution has to be exercised not to use positivity for these markers as evidence of squamous differentiation as non-squamous cell carcinomas which are of basal phenotype (ER, PR and HER2 negative) also tend to be positive for these above mentioned markers. These should be interpreted in the context of the morphology (7). Diagnosis of squamous differentiation is usually possible on morphology and supported by immune-stains for squamous markers. In our case, the tumour showed presence of intercellular bridges and keratin formation. If FNAC is done on these tumours, pultaceous cheesy material can be obtained in the aspirate which may be due to central area of keratinisation in the tumour. However in many instances, the keratin can elicit a foreign body granulomatous and inflammatory response which may mask the underlying atypical keratinised cells hence caution must be exercised in screening these smears or the diagnosis may be missed (3).
In general, metaplastic cancer, in contrast to IDC, tends to have fewer T1 tumours (30% vs. 65%), more node negative (78% vs. 66%) and less ER-positive tumours (11% vs. 74%) (10).These pathological features were also demonstrated in other studies (2,3), including our study which revealed a large triple-negative mass with no nodal involvement. Prognosis for this group of patients remains controversial with some studies reporting a poorer outcome (11) compared to the aggressive IDCs and other reports showing similar survival between the two groups (12). However, these reports were mainly confined to small retrospective case series.
Specifically, primary squamous cell breast cancer tends to present with a larger average tumour size of 5–10 cm compared to metaplastic squamous cell breast cancer of 2–5 cm (5). Cystic changes were also more common in primary squamous cell breast cancer (3,4). The extent of squamous differentiation is a determinant of disease free survival (DFS) with better prognosis for metaplastic carcinoma showing <40% squamous elements and worse for those with >90% squamous component (3,5). DFS and overall survival (OS) for metaplastic squamous cell breast cancer and primary squamous cell breast cancer were 64% versus 39.8% and 72.7% versus 66.7%, respectively (5).
There is little data on the optimal treatment regimen for metaplastic squamous cell breast cancer, and its management remains similar to that for IDCs (2). While review of the various treatment modalities has been reported for metaplastic squamous cell breast cancer, sparse reports have focused on surgical modality alone. We reviewed the available literature on surgical management in this rare group of patients and found that the majority of patients were treated with mastectomy and breast conservation was seldom used (3,13). There could be several contributing factors on why mastectomy was the reported surgery of choice in this group of patients, of which the rapid progression of the cancer, as objectively demonstrated in our case, and its poor response to neo-adjuvant conventional chemotherapy (3) could be important contributing factors. This hence highlights the importance of early treatment in this subtype of patients.
In conclusion, this case offered the first objective perception of the natural rapid progression of metaplastic squamous cell breast cancer if treatment was delayed and highlighted the importance of early treatment which could lead to the potential avoidance of mastectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Tavassoli FA. Classification of metaplastic carcinomas of the breast. Pathol Annu 1992;27:89-119. [PubMed]
- Graziano L, Graziano P. Metaplastic squamous cell carcinoma of the breast: A case report and literature review. Rev Assoc Med Bras (1992) 2016;62:618-21. [PubMed]
- Honda M, Saji S, Horiguchi S, et al. Clinicopathological analysis of ten patients with metaplastic squamous cell carcinoma of the breast. Surg Today 2011;41:328-32. [Crossref] [PubMed]
- Hennessy BT, Krishnamurthy S, Giordano S, et al. Squamous cell carcinoma of the breast. J Clin Oncol 2005;23:7827-35. [Crossref] [PubMed]
- Pai T, Shet T, Desai S, et al. Impact of Squamous Differentiation in Breast Carcinoma. Int J Surg Pathol 2016;24:483-9. [Crossref] [PubMed]
- Massuet A, Fernández S, Rimola J, et al. Metaplastic carcinoma of the breast: magnetic resonance and radiophatologic correlation. Radiologia 2006;48:155-63. [Crossref] [PubMed]
- Rakha EA, Coimbra ND, Hodi Z, et al. Immunoprofile of breast metaplastic carcinoma. Histopathology 2017;70:975-85. [Crossref] [PubMed]
- Reis-Filho JS, Milanezi F, Steele D, et al. Metaplastic breast carcinomas are basal-like tumours. Histopathology 2006;49:10-21. [Crossref] [PubMed]
- O'Malley FP, Pinder SE, Mulligan AM. Foundations in Diagnostic Pathology Series – Breast Pathology. Philadelphia: Elsevier, 2011.
- Pezzi CM, Patel-Parekh L, Cole K, et al. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol 2007;14:166-73. [Crossref] [PubMed]
- Schwartz TL, Mogal H, Papageorgiou C, et al. Metaplastic breast cancer: histologic characteristics, prognostic factors and systemic treatment strategies. Exp Hematol Oncol 2013;2:31. [Crossref] [PubMed]
- Gultekin M, Eren G, Babacan T, et al. Metaplastic breast carcinoma: a heterogeneous disease. Asian Pac J Cancer Prev 2014;15:2851-6. [Crossref] [PubMed]
- Albasri AM, Hussainy AS, Elkablawy MA, et al. Metaplastic squamous cell carcinoma of breast. A pathology case report with review of literature. J Pak Med Assoc 2015;65:785-7. [PubMed]