Concomitant thyroid disease and primary hyperparathyroidism in patients undergoing parathyroidectomy or thyroidectomy
Introduction
Thyroid nodules have a high prevalence (19–67%), with higher incidence in women and the elderly (1). Since 1973, the annual incidence of thyroid cancer (TC) has increased by more than 500% (2,3) and is expected to rise to 89,500 cases in 2019 (4). TC has the most rapid increase among all other types of cancers (4). The most common variant is well differentiated TC mainly papillary TC which accounts for approximately 95% of thyroid malignancies (5). The prevalence of primary hyperparathyroidism (PHPT) is only 0.1% in the general population (5); however, the incidence of PHPT is higher in patients with thyroid disease (0.3%) (6).
Few studies have examined the concomitant evidence of thyroid nodular disease and PHPT in patients undergoing parathyroidectomy (PTX) and thyroidectomy, but those that have shown high variability in coexistence of thyroid nodules and PHPT. The incidence of thyroid abnormalities in patients undergoing parathyroidectomy ranges from 16.6–84.3% with 4.0–88.0% undergoing thyroid procedures (5,7-30). Parathyroidectomy has also been reported in patients initially presenting with thyroid abnormalities who underwent thyroidectomy.
Surgical intervention is recommended for differentiated TC, certain benign thyroid nodules, and PHPT (1). Prior to ultrasound, thyroid abnormalities were found intraoperatively during parathyroidectomy and often resulted in a concomitant thyroidectomy (8,11,13,15,25). The identification of concomitant disease is important prior to primary operation in order to minimize surgical complications, patient discomfort, and costs. Surgical risks increase with reoperation as detection of the recurrent laryngeal nerve is more difficult as is preservation of parathyroid glands. One study comparing complication rates in primary thyroidectomy versus reoperation reported injury to the recurrent laryngeal nerve in 1.4% of patients undergoing thyroidectomy compared to 3% in the reoperation group. Rates of permanent hypocalcemia were also higher occurring in up to 3.5% of primary thyroidectomies and up to 5.9% of reoperations (31). This is due to increased scar tissue formation making visualization of structures more difficult (32,33). Therefore, efforts should be made to detect the coexistence of thyroid nodules and PHPT prior to thyroidectomy or parathyroidectomy, thereby decreasing the need for multiple surgeries (34).
The aim of this study is to determine the incidence of concomitant PHPT and thyroid nodular disease in patients undergoing thyroidectomy or parathyroidectomy.
Methods
This was a retrospective review of prospectively gathered data for 621 patients who underwent thyroidectomy, parathyroidectomy, or both by a single surgeon, the senior author, at Tulane Medical Center between January 2012 and June 2016. Patients with multiple endocrine neoplasia, familial hyperparathyroidism (HPT), or history of total thyroidectomy were excluded.
Data was collected retrospectively from patient charts with institutional IRB approval. Information obtained included patient age, gender, reason for initial referral, history of irradiation, calcium level, initial thyroid stimulating hormone (TSH), initial parathyroid hormone (PTH), fine needle aspiration (FNA) results, ultrasound results, type of operation performed, final diagnosis, and final pathology.
Descriptive statistics were performed for the entire sample. Bivariate analysis based on treatment versus control was conducted with independent sample t-test comparing means and Wilcoxon rank sum tests comparing medians for continuous variables as appropriate, and Pearson’s chi-square test or Fisher’s exact test comparing proportions for categorical variables as appropriate. Multivariable logistic linear regression was conducted based on significant bivariate results. Statistical significance was set at two-tailed P value <0.05. All analyses were conducted using Stata 14.2 (StataCorp, College Station, Texas, USA). Approval for this study was obtained from Tulane University under IRB number 686910-1.
Results
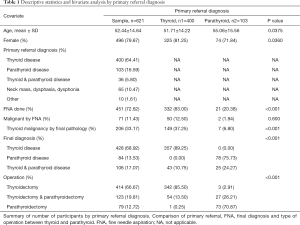
Of the 621 participants, 496 were women and 125 were men, with a mean age of 52.44 years. Of these, 400 patients were referred primarily for thyroid disease, 103 for parathyroid disease, 36 patients were referred for both thyroid and parathyroid disease, 75 patients were referred for neck masses, or compressive symptoms. For the purpose of our study, patients referred for both thyroid disease or parathyroid disease and neck mass, dysphasia, or dysphonia were included under thyroid or parathyroid disease referrals.
Among the 400 patients who were referred primarily for thyroid disease, 54 (13.50%) underwent a thyroidectomy and PTX simultaneously, while 342 (85.50%) patients underwent a thyroidectomy only. Of the 400 patients, 357 (89.25%) were given a final diagnosis of thyroid disease and 43 (10.75%) received a final diagnosis of thyroid and concomitant parathyroid disease. FNA was performed for 332 (83.00%) of the patients and 50 (12.50%) had FNA results suggestive of thyroid malignancy. However, 149 patients (37.25%) were diagnosed with a thyroid malignancy based on the final pathology report (Table 1).
Full table
Among the 103 patients referred primarily for parathyroid disease, 27 (26.21%) underwent a PTX and thyroidectomy, 73 (70.87%) underwent a PTX only, and 3 (2.91%) underwent a thyroidectomy. Of these patients, 78 (75.73%) received a final diagnosis of parathyroid disease only while 25 (24.27%) received a final diagnosis of both thyroid and parathyroid disease. FNA was performed for 21 (20.39%) of these patients and 2 (1.94%) had FNA results suggestive of thyroid malignancy. Seven (6.80%) were found to have thyroid malignancy (Table 1).
Without considering primary referral diagnosis, 451 (72.62%) patients had a FNA done and of these 70 (15.74%) had an FNA confirmed malignancy while 192 (40.17%) were benign. Five hundred and seventy-five patients underwent a neck ultrasound. Of these 59 (10.26%) had no nodule or a nodule less than 1 cm while 454 (78.96%) had a nodule greater than or equal to 1 cm and 43 (7.48%) had parathyroid disease. Descriptive statistics are summarized in Table 1.
Controlling for age, patients referred primarily for parathyroid disease were more likely to receive a final diagnosis of both parathyroid and thyroid disease (OR =2.42, P=0.002). Additionally, patients referred primarily for parathyroid disease were more likely to undergo a combined operation than a single operation (OR =2.03, P=0.010) (Table 2).

Full table
Discussion
Concomitant thyroid and parathyroid disease has been documented in several studies with the first report by Hellstrom in 1954. Of 50 patients evaluated for PHPT, 19 patients had thyroid nodular disease (5,35). Two years later, Ogburn and Black reported that of 230 patients who underwent surgery for PHPT, four patients were found to have differentiated TC (5,36). Similar studies between 1982 and 2007 reported TC in patients undergoing PTX ranging from 2.1–17.6% (5,8,14-18,37-39). For these studies, concomitant disease was discovered intraoperatively. The first study to look at pre-operative analysis of concomitant disease was performed by Morita et al. in 2008. Our results were consistent with those findings: 3.45% of patients referred for parathyroid disease were found to have TC on final pathology.
Advanced preoperative imaging and surgical techniques introduced in the 1990s have decreased bilateral neck exploration. Technetium-99m sestamibi (MIBI) and improved ultrasound technology have made unilateral neck exploration an option in cases of PHPT due to a single adenoma (20). Additionally, endoscopic PTX offers a less invasive surgical option for simple PTX (20,40). While these minimally invasive surgical procedures provide more cosmetically desirable results, they limit exploration and visualization of other neck structures. In these cases, pre-operative screening for concomitant thyroid and parathyroid disease becomes even more important.
HPT should also be considered in patients with thyroid disease. In a study of 13,000 patients who presented with thyroid disease, PHPT was detected in 0.3% of patients. However, a more recent study by Morita et al. found PHPT in 3.1% of patients referred for thyroid disease. We found that 10% of patients referred for thyroid disease received a final diagnosis of thyroid and parathyroid disease.
As demonstrated by our study and similarly conducted trials, concomitant thyroid and parathyroid disease can occur. Previous studies suggest the risk of thyroid disease is higher in patients with PHPT. Additionally, parathyroid disease is more prevalent in patients with thyroid disease. A study by Murray et al. found that 5% of patients undergoing thyroidectomy also underwent PTX (34). Additionally, concomitant thyroid disease and PHPT can result in complications for patients with untreated hypercalcemia, unrecognized TC, or additional neck surgery (5). Patients presenting with thyroid disease should undergo analysis of serum calcium and possibly PTH levels to check for underlying parathyroid disease. Patients presenting with PHPT should undergo neck ultrasound to detect thyroid nodules and subsequent FNA if indicated to assess for malignancy. Detection of concomitant disease pre-operatively can help guide surgical planning, decrease post-operative complications of undetected disease, and minimize the need for reoperation.
However, while there is certainly a role for imaging in thyroid and parathyroid disease detection and management, recent studies have suggested that increased imaging may be responsible for a higher incidence of papillary TC. The incidence of well-differentiated TC in the US has increased from 4.9 per 100,000 in 1975 to 14.3 per 100,000 in 2009 (41). This may be due to detection of subclinical disease, as autopsy reports show TC in people who have died from non-thyroid related diseases (41). The question becomes how to treat these cases of TC, especially low risk papillary carcinomas. As described above, the default approach for thyroid carcinoma has been thyroidectomy. However, recent studies have suggested that lobectomy may be a more conservative approach which produces similar results. A study from the SEER database showed no survival benefit for patients with low risk tumors who were treated with a more aggressive surgical approach. Less invasive options for low risk papillary TC are being studied. Radiofrequency ablation and ultrasound guided ethanol ablation have been used, but their efficacy is not well documented. Two Japanese studies compared thyroidectomy and active surveillance and found that at 5 years 1.6% of patients had lymph node metastasis and 6.4% had asymptomatic lesion growth. This increased to 3.4% and 15.9% at 10 years (42) suggesting that not all low risk TC requires immediate surgical intervention.
A cost-effectiveness comparison looking at early surgery versus a non-surgical approach (NSA) for incidental papillary thyroid microcarcinoma found that a NSA was less costly for patients who were elderly or had a life expectancy ≤16 years (43). However, when looking only at cost, a surgical approach was preferable for patients with a life expectancy of 17 or more years, i.e., healthier and younger patients. When measuring effectiveness in terms of quality-adjusted life years (QALYs), the study found NSA more effective (0.260 QALY) regardless of life expectancy, as NSA resulted in fewer surgery-related permanent complications such as vocal cord palsy and hypoparathyroidism (43). Post sensitivity analysis, Lang and Wong determined that NSA, or observation, was always more cost-effective in managing incidental papillary thyroid microcarcinoma, although the authors admitted a significant portion of patients and physicians may prefer surgical intervention due to anxiety and uncertainty. The authors also mentioned that their model assumed a constant rate of progression and spread, and selection bias was possible, as patients opting for NSA may have had disease that was less likely to progress or spread (43).
When looking at concomitant thyroid and parathyroid disease it is important to weigh the pros and cons of diagnosis and intervention. There has been a large increase in the incidence of thyroid disease discovered as a result of advances in imaging. While screening for thyroid disease with ultrasound plays an important role in disease detection and treatment, it is important to determine the implications of disease diagnosis. Perhaps the increased incidence of low risk TC is due to subclinical detection that can be managed with more conservative treatment. However, there have been no studies comparing lobectomy vs. total thyroidectomy vs. non-surgical treatment for low risk thyroid carcinoma. This may be an important area of research to determine the best management of incidentally discovered thyroid carcinoma.
Acknowledgements
We would like to thank H Mohamed, MD for his guidance editing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Data was collected retrospectively from patient charts with institutional IRB approval. Approval for this study was obtained from Tulane University under IRB number 686910-1.
References
- Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Lubitz CC, Kong CY, McMahon PM, et al. Annual financial impact of well-differentiated thyroid cancer care in the United States. Cancer 2014;120:1345-52. [Crossref] [PubMed]
- US National Cancer Institute. Surveillance, Epidemiology, and End Results Program. 2015. Available online: https://seer.cancer.gov/statistics/summaries.html
- Aschebrook-Kilfoy B, Schechter RB, Shih YC, et al. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomarkers Prev 2013;22:1252-9. [Crossref] [PubMed]
- Morita SY, Somervell H, Umbricht CB, et al. Evaluation for concomitant thyroid nodules and primary hyperparathyroidism in patients undergoing parathyroidectomy or thyroidectomy. Surgery 2008;144:862-6; discussion 866-8. [Crossref] [PubMed]
- Wagner B, Begic-Karup S, Raber W, et al. Prevalence of primary hyperparathyroidism in 13387 patients with thyroid diseases, newly diagnosed by screening of serum calcium. Exp Clin Endocrinol Diabetes 1999;107:457-61. [Crossref] [PubMed]
- Ellenberg AH, Goldman L, Gordan GS, et al. Thyroid carcinoma in patients with hyperparathyroidism. Surgery 1962;51:708-17. [PubMed]
- Prinz RA, Barbato AL, Braithwaite SS. Simultaneous primary hyperparathyroidism and nodular thyroid disease. Surgery 1982;92:454-8. [PubMed]
- Kambouris AA, Ansari MR, Talpos GB. Primary hyperparathyroidism and associated neoplasms. Henry Ford Hosp Med J 1987;35:207-10. [PubMed]
- Strichartz SD, Giuliano AE. The operative management of coexisting thyroid and parathyroid disease. Arch Surg 1990;125:1327-31. [Crossref] [PubMed]
- Attie JN, Vardhan R. Association of hyperparathyroidism with nonmedullary thyroid carcinoma: Review of 31 cases. Head Neck 1993;15:20-3. [Crossref] [PubMed]
- Kairaluoma MV. Simultaneous thyroid operation in patients undergoing initial neck exploration for primary hyperparathyroidism. Ann Chir Gynaecol 1994;83:30-4. [PubMed]
- Krause UC, Friedrich JH, Olbricht T, et al. Association of primary hyperparathyroidism and non-medullary thyroid cancer. Eur J Surg 1996;162:685-9. [PubMed]
- Sidhu S, Campbell P. Thyroid pathology associated with primary hyperparathyroidism. Aust N Z J Surg 2000;70:285-7. [Crossref] [PubMed]
- Bentrem DJ, Angelos P, Talamonti MS, et al. Is preoperative investigation of the thyroid justified in patients undergoing parathyroidectomy for hyperparathyroidism? Thyroid 2002;12:1109-12. [Crossref] [PubMed]
- Beus KS, Stack BC. Synchronous thyroid pathology in patients presenting with primary hyperparathyroidism. Am J Otolaryngol 2004;25:308-12. [Crossref] [PubMed]
- Kösem M, Algün E, Kotan C, et al. Coexistent thyroid pathologies and high rate of papillary cancer in patients with primary hyperparathyroidism: controversies about minimal invasive parathyroid surgery. Acta Chir Belg 2004;104:568-71. [Crossref] [PubMed]
- Masatsugu T, Yamashita H, Noguchi S, et al. Thyroid evaluation in patients with primary hyperparathyroidism. Endocr J 2005;52:177-82. [Crossref] [PubMed]
- Kairys JC, Daskalakis C, Weigel RJ. Surgeon-performed ultrasound for preoperative localization of abnormal parathyroid glands in patients with primary hyperparathyroidism. World J Surg 2006;30:1658-63; discussion 1664.
- Ogawa T, Kammori M, Tsuji EI, et al. Preoperative evaluation of thyroid pathology in patients with primary hyperparathyroidism. Thyroid 2007;17:59-62. [Crossref] [PubMed]
- Zheng YX, Xu SM, Wang P, et al. Preoperative localization and minimally invasive management of primary hyperparathyroidism concomitant with thyroid disease. J Zhejiang Univ Sci B 2007;8:626-31. [Crossref] [PubMed]
- Monroe DP, Edeiken-Monroe BS, Lee JE, et al. Impact of preoperative thyroid ultrasonography on the surgical management of primary hyperparathyroidism. Br J Surg 2008;95:957-60. [Crossref] [PubMed]
- Siperstein A, Berber E, Barbosa GF, et al. Predicting the success of limited exploration for primary hyperparathyroidism using ultrasound, sestamibi, and intraoperative parathyroid hormone: analysis of 1158 cases. Ann Surg 2008;248:420-8. [PubMed]
- Gates JD, Benavides LC, Shriver CD, et al. Preoperative thyroid ultrasound in all patients undergoing parathyroidectomy? J Surg Res 2009;155:254-60. [Crossref] [PubMed]
- Heizmann O, Viehl CT, Schmid R, et al. Impact of concomitant thyroid pathology on preoperative workup for primary hyperparathyroidism. Eur J Med Res 2009;14:37-41. [PubMed]
- Norman J, Politz D. 5,000 parathyroid operations without frozen section or PTH assays: measuring individual parathyroid gland hormone production in real time. Ann Surg Oncol 2009;16:656-66. [Crossref] [PubMed]
- Adler JT, Chen H, Schaefer S, et al. Does Routine Use of Ultrasound Result in Additional Thyroid Procedures in Patients with Primary Hyperparathyroidism? J Am Coll Surg 2010;211:536-9. [Crossref] [PubMed]
- Onkendi EO, Richards ML, Thompson GB, et al. Thyroid Cancer Detection with Dual-isotope Parathyroid Scintigraphy in Primary Hyperparathyroidism. Ann Surg Oncol 2012;19:1446-52. [Crossref] [PubMed]
- Arciero CA, Shiue ZS, Gates JD, et al. Preoperative thyroid ultrasound is indicated in patients undergoing parathyroidectomy for primary hyperparathyroidism. J Cancer 2012;3:1-6. [Crossref] [PubMed]
- Kwon JH, Kim EK, Lee HS, et al. Neck ultrasonography as preoperative localization of primary hyperparathyroidism with an additional role of detecting thyroid malignancy. Eur J Radiol 2013;82:e17-21. [Crossref] [PubMed]
- Vaiman M, Nagibin A, Olevson J. Complications in Primary and Completed Thyroidectomy. Surg Today 2010;40:114-8. [Crossref] [PubMed]
- Alesina PF, Rolfs T, Hommeltenberg S, et al. Intraoperative neuromonitoring does not reduce the incidence of recurrent laryngeal nerve palsy in thyroid reoperations: results of a retrospective comparative analysis. World J Surg 2012;36:1348-53. [Crossref] [PubMed]
- Pironi D, Pontone S, Vendettuoli M, et al. Prevention of complications during reoperative thyroid surgery. Clin Ter 2014;165:e285-90. [PubMed]
- Murray SE, Sippel RS, Chen H. Incidence of concomitant hyperparathyroidism in patients with thyroid disease requiring surgery. J Surg Res 2012;178:264-7. [Crossref] [PubMed]
- HELLSTROM J. Primary hyperparathyroidism; observations in a series of 50 cases. Acta Endocrinol (Copenh) 1954;16:30-58. [PubMed]
- Ogburn PL, Black BM. Primary hyperparathyroidism and papillary adenocarcinoma of the thyroid; report of four cases. Proc Staff Meet Mayo Clin 1956;31:295-8. [PubMed]
- Milas M, Mensah A, Alghoul M, et al. The impact of office neck ultrasonography on reducing unnecessary thyroid surgery in patients undergoing parathyroidectomy. Thyroid 2005;15:1055-9. [Crossref] [PubMed]
- Fedorak IJ, Salti G, Fulton N, et al. Increased incidence of thyroid cancer in patients with primary hyperparathyroidism: a continuing dilemma. Am Surg 1994;60:427-31. [PubMed]
- Hedman I, Fjälling M, Lindberg S, et al. An assessment of the risk of developing hyperparathyroidism and thyroid disorders subsequent to neck irradiation in middle-aged women. J Surg Oncol 1985;29:78-81. [Crossref] [PubMed]
- Kwak HY, Kim SH, Chae BJ, et al. Learning curve for gasless endoscopic thyroidectomy using the trans-axillary approach: CUSUM analysis of a single surgeon’s experience. Int J Surg 2014;12:1273-7. [Crossref] [PubMed]
- Brito JP, Hay ID, Morris JC. Low risk papillary thyroid cancer. BMJ 2014;348:g3045. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Inoue H, et al. An Observational Trial for Papillary Thyroid Microcarcinoma in Japanese Patients. World J Surg 2010;34:28-35. [Crossref] [PubMed]
- Lang BH, Wong CK. A cost-effectiveness comparison between early surgery and non-surgical approach for incidental papillary thyroid microcarcinoma. Eur J Endocrinol 2015;173:367-75. [Crossref] [PubMed]


