Microsurgical refinements with the use of internal mammary (IM) perforators as recipient vessels in transverse upper gracilis (TUG) autologous breast reconstruction
Introduction
Immediate breast reconstruction has the advantages of reducing the number of operations, reduced psychological morbidity, with better aesthetic outcomes as the skin envelope is preserved (1).
Autologous breast reconstruction in thin patients presents a challenging problem. Thin patients are not considered ideal candidates for autologous reconstructions due to the lack of adequate adiposity (2).
When making a decision about the choice of reconstruction, there are certain aspects that need to be considered. The patients over all fitness for surgery, any pre-operative radiotherapy, need for adjuvant therapy and the patients preference on the method of reconstruction after being fully educated on the available options.
Autologous breast reconstructions have become the gold standard in most plastic surgery units, it provides an aesthetically pleasing and long lasting natural reconstruction (3-7)
The DIEP flap has become the flap of choice in both unilateral and bilateral breast reconstructions. When the DIEP flap is not available, the second choice would either be a unilateral or bilateral transverse upper gracilis (TUG) flap. TUG flaps provide durable, pliable tissue for breast reconstruction with well hidden scars.
In this paper we will discuss the technique of IMAP preparation the advantages and disadvantages of the IMA perforator in TUG flap reconstructions together with methods of correcting vessel size mismatch and potential methods to prevent/reduce the complication of venous coupler.
Methods
Computerised records of all patients undergoing TUG flap reconstructions involving the IMA perforators at St. Andrews Centre of Plastic Surgery between March 2015 and March 2016 were retrospectively reviewed. Patient demographics, the number of TUG flaps/ IMAP used, TUG flap weight, size of IMAP/IMVP vessel size, the size of the venous couplers used and complications were recorded.
Results
Over the 1 year period, 12 patients underwent 14 TUG flaps, 10 unilateral TUG flaps (8 for unilateral reconstruction post mastectomy and 2 for partial breast defects), 1 bilateral reconstruction and 1 bilateral TUG for a unilateral reconstruction. Average age was 53.25, range from 45 to 65. Average TUG weight was 320 grams with a range from 219 to 585 grams. Thirteen IMAP were used with an average IMVP size of 2.19 and average IMAP size of 1.65.
The average coupler size used was 2.11 (5 with 2.5 mm, 6 with 2 mm and 2 with 1.5 mm).
Of the 12 cases there was one partial flap necrosis that required debridement and re-advancement of the flap, one case of donor site seroma which settled with multiple aspirations in the outpatient department and 2 cases of palpable/tender venous coupler both being 2.5 mm in size.
Discussion
The TUG flap is our second choice for autologous reconstruction when the DIEP flap is not available. When comparing it to the SGAP/IGAP flaps it provides more pliable tissue for breast reconstruction, it is a relatively straightforward flap to raise with the patient in a supine position. This is offset with a short pedicle, a higher donor site complication profile with wound breakdown, sensory disturbances, poor scarring and prolonged seroma formation (8,9).
Various recipient vessels are available, IMA perforator, internal mammary (IM) vessels, IM perforators or thoracodorsal/serratus vessels. The IMA can be accessed by either removing a small section of costal cartilage or via an intercostal space technique. A rib-sparing technique which allows for a longer length of recipient vessel has been described by Hunter et al. This involves temporarily excising one of the costal cartilages in the subperichondrial plane. Once the IM vessels have been dissected from the rib below to the rib above the rib cartilage is replaced into its perichondrial lining.
With the use of the thoracodorsal vessel or serratus branch a vein graft is often needed to allow medialisation of the TUG flap.
The IMA perforator is our preferred recipient vessel during TUG flap reconstruction. The advantage of reducing morbidity and overall dissection time harvesting the IMAP vessels is offset by a more frequent diameter mismatch between the vessels used.
Preparation of the perforator
In this series the IMA perforator either the 2nd or 3rd was used to anastomose to the TUG vessels. It is critical that these vessels are not damaged during the mastectomy and their rough position should be marked prior to the mastectomy to prevent inadvertent damage. In some cases damage does occur but if at least 1cm of vessel above the pectoralis fascia is available this can still be used safely.
Once the perforator is identified in the mastectomy skin distally it is advisable to dissect this off the mastectomy skin from distal to proximal. Attempting initial dissection proximally around the level of the pectoralis fascia can result in damage. Once the IMAP is dissected to the level of the pectoralis major muscle fascia, the muscle is carefully split to expose the vessel further. The artery size is then assessed if this is equal or greater then 1mm it is suitable. A single clamp is then placed at the base of the vessels and the vessels shortened to the level required. The rest of the preparation proceeds under the microscope.
Vessel mismatch
In the recent years progress in the manufacture of optical devices and microsurgical instruments enable to perform micro sutures in vessels smaller than a millimetre in diameter. Nevertheless vessels diameter mismatch remains a challenge even for most experienced micro surgeons.
While the vein size discrepancy is well managed with the use of the anastomotic coupler device (10), but the approach to arterial vessels is different.
Diameter mismatch of 1:1.5
This is usually managed with a gentle dilatation of the smaller vessel with the vessels dilator.
Diameter mismatch of 1:2
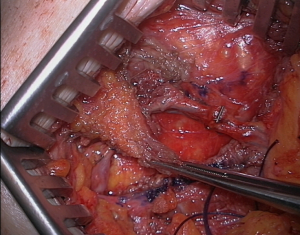
Technique 1: we use a modification of the Harashina’s fish-mouth technique (11). Figure 1 shows the double wedge excision fish-mouth technique: two opposite triangle are longitudinally excised from IMAP artery and a gentle dilatation applied to expand the diameter of the vessel allowing for the anastomosis to be performed.

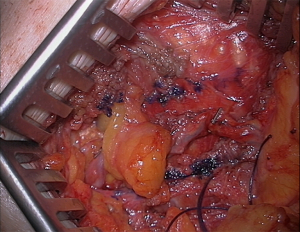
Technique 2: if during recipient vessel dissection a bifurcation or branch of the IMAP artery is noticed this can be used to minimize the size discrepancy. In Figure 2, it shows this technique: after adventitia removal the two branches are excised close to the bifurcation, the open tip of the micro scissors is passed from one branch to the other and the bifurcation opened. The excess portion of vessel is trimmed and the diameter increase permits anastomosis of the vessel in the usual fashion.

Overcoming problem with a palpable/tender venous coupler
In our series, we identified 2 patients who had a palpable tender venous coupler, in both cases the coupler was 2.5 mm in size. As most patients who undergo TUG reconstructions and relatively slim this is a significant problem and we suggest potential methods to prevent/reduce this complication which we have adopted to try and prevent/reduce the incidence of this complication especially when 2.5 mm couplers or greater are used.
- Placing the coupler away from a prominent rib and therefore reducing its prominence.
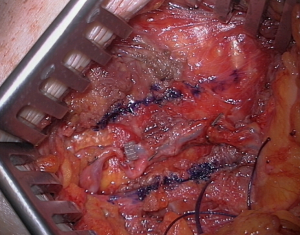
- Creating a channel within the pectoralis major muscle and therefore allowing the coupler to lie flush with the muscle (Figure 3).
- Covering the coupler with a flap of pectoralis muscle (Figure 4) or piece of fat (Figure 5).
Medialisation
With the use of the IMA perforators as the recipient vessels, this allows the TUG flap to be placed in the ideal position giving a superior aesthetic result. However with ptotic breasts where the breast sits low on the chest wall the 2nd IM perforator may medialise the flap excessively. We recommend the use of the 3rd IM perforator in this case or extension of the TUG pedicle on bench with a vein graft. If the thoracodorsal vessels/serratus branch is used it is difficult to medialise the flap without the use of a vein graft.
Vein graft
During flap raising a medial tributary of the long saphenous is divided close to the main vessel. This vein usually runs horizontally along the length of the inferior aspect of the flap and can be a valuable source for a vein graft.
Conclusions
The TUG flap is our second choice for breast reconstruction when the DIEP flap is not available. The short pedicle length and limited flap volumes makes this a reconstructive challenge. In this paper we described our experience with using the IMAP vessels for primary anastomosis. The IMAP makes an ideal donor vessel as it is relatively straightforward to dissect and prepare. The vessel mismatch can be somewhat of a challenge but the two techniques described in this article will help to overcome this. The IMAP vessels are our preferred donor vessels for TUG flap reconstructions. These refinements would help to further the aesthetic outcome of TUG flap reconstructions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This article does not require ethical approval.
References
- Dean C, Chetty U, Forrest AP. Effects of immediate breast reconstruction on psychosocial morbidity after mastectomy. Lancet 1983;1:459-62. [Crossref] [PubMed]
- Kitcat M, Molina A, Meldon C, et al. A Simple Algorithm for Immediate Postmastectomy Reconstruction of the Small Breast-A Single Surgeon's 10-Year Experience. Eplasty 2012;12:e55. [PubMed]
- Garvey PB, Villa MT, Rozanski AT, et al. The advantages of free abdominal-based flaps over implants for breast reconstruction in obese patients. Plast Reconstr Surg 2012;130:991-1000. [Crossref] [PubMed]
- Mehrara BJ, Santoro TD, Arcilla E, et al. Complications after microvascular breast reconstruction: experience with 1195 flaps. Plast Reconstr Surg 2006;118:1100-9; discussion 1110-1. [Crossref] [PubMed]
- Alderman AK, Kuhn LE, Lowery JC, et al. Does patient satisfaction with breast reconstruction change over time? Two-year results of the Michigan Breast Reconstruction Outcomes Study. J Am Coll Surg 2007;204:7-12. [Crossref] [PubMed]
- Rosson GD, Magarakis M, Shridharani SM, et al. A review of the surgical management of breast cancer: plastic reconstructive techniques and timing implications. Ann Surg Oncol 2010;17:1890-900. [Crossref] [PubMed]
- Yueh JH, Slavin SA, Adesiyun T, et al. Patient satisfaction in postmastectomy breast reconstruction: a comparative evaluation of DIEP, TRAM, latissimus flap, and implant techniques. Plast Reconstr Surg 2010;125:1585-95. [Crossref] [PubMed]
- Arnez ZM, Pogorelec D, Planinsek F, et al. Breast reconstruction by the free transverse gracilis (TUG) flap. Br J Plast Surg 2004;57:20-6. [Crossref] [PubMed]
- Locke MB, Zhong T, Mureau MA, et al. Tug 'O' war: challenges of transverse upper gracilis (TUG) myocutaneous free flap breast reconstruction. J Plast Reconstr Aesthet Surg 2012;65:1041-50. [Crossref] [PubMed]
- Fitzgerald O'Connor E, Rozen WM, Chowdhry M, et al. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland Surg 2016;5:88-92. [PubMed]
- Harashina T, Irigaray A. Expansion of smaller vessel diameter by fish-mouth incision in microvascular anastomosis with marked size discrepancy. Plast Reconstr Surg 1980;65:502-3. [Crossref] [PubMed]
- Saour S, Libondi G, Ramakrishnan V. Shows the double wedge excision fish-mouth technique. Asvide 2017;4:309. Available online: http://www.asvide.com/articles/1621
- Saour S, Libondi G, Ramakrishnan V. Technique of increasing vessel diameter using the bifurcation or branch of the IMAP artery. Asvide 2017;4:310. Available online: http://www.asvide.com/articles/1622