Gasless trans-axillary robotic thyroidectomy: the introduction and principle
The concept of endoscopic neck surgery for thyroid or parathyroid gland started with Gagner et al. in 1996 (1), where the first case of endoscopic parathyroidectomy was reported. Soon after, in 1997, the first case of thyroid lobectomy via endoscopic approach was reported by Hüscher et al. (2). Since then, the mini-invasive concept for thyroid surgeries has started growing, and many variations of the endoscopic technique have appeared.
The endoscopic era
Endoscopic surgery can be divided into two main types, the direct approach (cervical), and the indirect approach (extra-cervical) (3). The concept of the direct approach is doing smaller neck incision, with direct exposure of the thyroid gland while using endoscopic instruments. The indirect approach is moving the incision out of the cervical region, to the axilla, or breast, or retro-auricular and exposing the thyroid gland from a lateral point of view (Figure 1).
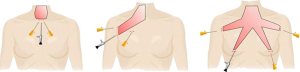
The two essential examples are the minimally invasive video-assisted thyroidectomy (MIVAT) and the minimally invasive lateral approach. The MIVAT is a technique developed by Miccoli et al. in Italy in 1998 (4). The main advantage of this technique is the good cosmetic result (5,6), and its main inconvenient is the limited indications (small glands volume <25 mL). The endoscopic lateral approach is another technique that requires CO2 insufflation. The crucial weak point is that the surgeon can only resect the ipsilateral lobe.
The principal purpose of the endoscopic thyroid surgery is to improve cosmetic results, as expected due to improved visualization (magnified vision), in order to decrease the rate of complications with a better identification of the important structures, the recurrent laryngeal nerve and the parathyroid glands.
When using direct endoscopic technique, a neck scar is always present. It might be smaller or lateralized, but it is still there, hence, the importance of the indirect endoscopic thyroidectomy.
The extra cervical approach can be divided into two main groups, the chest/breast and trans-axillary, and the combination of them both. The first approach reported is the infraclavicular by Shimizu et al. in 1998 (7). The basic problems encountered are the visible scar (not well hidden by clothes) and the risk of hypertrophic scarring. As a result, Ohgami et al. developed in 2000, another extra-cervical approach, characterized by being more hidden: the breast approach (8).
For total thyroidectomy, a bilateral breast incision is made. CO2 insufflation is needed, and there is always an extra concern for patients with breast implants. The trans-axillary approach was first reported by Ikeda et al. in 2000 (9). This technique provides a remarkable cosmetic result, with a well-hidden scar in the axillary region. The distance to the thyroid gland is not very important; therefore, less subcutaneous dissection is performed and the breast area is spared. Also, no CO2 insufflation is needed.
Two trocars are placed through the axillary incision, but one more is needed on the chest area. Total thyroidectomy can be performed from a single axillary incision, the contralateral lobe can be approached from the medial side, but it is a difficult due to the small working space and the instruments’ collision. Some reported lowering the anterior chest incision to the breast level, in order to avoid hypertrophic or keloid scars (unilateral axillo-breast approach) (10). Some recent articles described the approach with only a single axillary incision (11,12). The axillo-bilateral-breast approach was introduced by Shimazu et al. to overcome the narrow view and limited mobility (13). It requires wider dissection and CO2 insufflation.
Later on, Choe et al. added a contralateral axillary incision, called the technique bilateral axillo-breast (BABA) approach (14). Due to the wide dissection needed and the post-operative chest discomfort, some authors qualified this technique by “maximally” invasive (15,16). Lee et al. described another technique, the post auricular and axillary (PAA) approach (17), to avoid incision in the breast area. It needs CO2 insufflation, and may expose some facial nerve branches to stretching.
Those were the main endoscopic approaches to thyroid surgery. However, endoscopic thyroidectomy procedures are generally too long and technically demanding to be adopted on a large scale (18). The complexity of the endoscopic thyroid surgery makes it only available in highly specialized surgical centers. These aforementioned different techniques have some similar difficulties, such as the shared instruments with laparoscopic surgery and the limited degree of freedom, hence making the dissection of delicate structures like the recurrent laryngeal nerve, and parathyroid glands challenging. In some other limitations, the images are two dimensional and unstable, the endoscope being held by the assistant. Techniques that require CO2 insufflation can cause some serious complications, such as hypercapnia, gas embolism, respiratory acidosis and subcutaneous emphysema.
The robotic era
These technical disadvantages have fueled the need to upgrade the endoscopic technique. All that led to the introduction of the robot to the thyroid surgery. A 3-dimensional stable and magnified image, instruments with seven degrees of freedom, gasless technique… are some of the crucial advantages of the addition of robot to surgery, especially thyroid and neck surgery, interventions with small working space. In fact, in 2009, the team of Professor Chung introduced the transaxillary robotic thyroidectomy (19).
The history of the robotic surgery goes back to many years ago. The word “robot” appeared a century ago, precisely in 1921, in a play entitled “Rossum’s Universal Robots” written by Czech writer Karel Čapek (Figure 2). Ever since, the robot uses have expanded to many fields, especially to the industrial domain.

Concerning the medical field, the constant need to improve the precision, mainly in surgery, led to the development of special devices. The first ever was PUMA 560, developed in 1985, to help improve precision of position in CT guided neurosurgical procedures (21) (Figure 3). Its development led to the creation of PROBOT in the late 1980’s (23) (Figure 4). “Integrated Surgical Supplies Ltd.” of Sacramento, CA, USA, developed in 1992 ROBODOC, a robot used in hip replacement surgeries (25) (Figure 5). It was the first robot to be FDA approved.

The next big thing in the history of robots in the surgical field was the FDA approval of the AESOP 1000 (Automated Endoscopic System for Optimal Positioning by Computer Motion, Santa Barbara, CA, USA) as a camera holder (27) (Figure 6). Actually, Computer Motion is the end result of conjoint efforts from NASA, the Stanford Research Institute and the US army. The purpose of NASA was to develop telesurgery and that of the US army was to decrease wartime mortality by “bringing the surgeon to the wounded soldier through telepresence” (29). So, AESOP was the civilian result of these researches. Zeus became available in 1998, and introduced the idea of telesurgery (Figure 7).

Meanwhile, the Green Telepresence Surgery System was developed with the goal of improving surgical capabilities on the battlefield. This led to the creation of Intuitive Surgical. After many legal battles, Computer motion and Intuitive Surgical merged in 2003; the development of ZEUS was dropped and the concentration on da Vinci increased.
The da Vinci® robot is the most used robot system in the medical field. The cardiac surgery was the primary goal in its development. However, da Vinci® became famous and most known for its use in general surgery, urology and gynecology (30). Da Vinci® was FDA approved in 2000 for abdominal surgeries. It is a system based on the formula of “Master-Slave” relation. The surgeon placed at his console, controls the arms of the robot using joysticks that filter any hand tremor and copy, by a scaled motion, the movement of the operator’s hands. One of the main highlights is the 3-dimensional, magnified and binocular vision. There is no need for special glasses to see in 3D mode. The high mobility of the instruments (seven degrees of freedom) and the improved ergonomic with conservation of the natural eye hand instrument alignment, are important features (Figures 8,9).
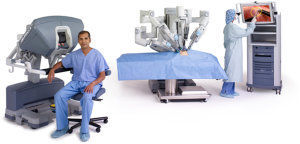
Transaxillary robotic thyroidectomy
However, it was until 2007, when Professor Chung did the first gasless robotic transaxillary thyroidectomy and published the experience of the first 100 cases in 2009 (19). The added value of robotic surgery over endoscopic in the thyroid field has led to a spread of this technique.
Whether it was the German Professor Dr. Erich Mühe in 1985, or the French surgeon Dr. Phillipe Mouret in 1987, who performed the first laparoscopic cholecystectomy (according to the book of Litynski GS, and the paper of Reynolds W Jr) (32,33), this event was the real kick-off of the minimally invasive surgery. At first, the surgical society was skeptical towards this concept. Shortly after, it became a very accepted and wanted approach from surgeons and patients. Less dissection, less scars, less operative time, less hospital stay… are all criteria that surgeons and patients at the same time search and tend for. And to overcome the limits of endoscopic technique, came the robotic surgery.
The neck is generally considered a favorable location for keloid and hypertrophic scars, mainly in Asian and African populations. The presence of a scar in the neck is not very well accepted in some societies. This is one of the reasons that led to the development of extra-cervical approaches for thyroidectomy. In addition, the major proportion of the patients is young females.
Some studies have shown that the size of the cervical incision and thus the scar, is not related to the patient satisfaction (34). And when the decision is given to the patients, they prefer a scarless neck (35), noting that patients have better self-body image after robotic transaxillary thyroid surgery. It also improves quality of life (36), and patients have a better satisfaction with the neck appearance (37). Further to the cosmetic up hand, the transaxillary robot assisted thyroidectomy has shown to be less invasive to the neck muscles. Patients tend to have lesser swallowing difficulties, and less neck discomfort (38). Robotic surgery is also less invasive on the voice, probably due to the better visualization and the more delicate dissection of the recurrent laryngeal nerve, and even on long term (up to 2 years) it provides better recovery of voice symptoms and acoustic parameters (39).
Many reviews and meta-analysis made compared the robotic thyroid surgery, and mainly the transaxillary approach, to the endoscopic or the conventional technique. Robot surgery is as safe as the classic technique, and comparable surgical completeness in carcinomas (differentiated) were proved. A shorter hospital stay was also found. Comparable rates of complications were found, but lower risk of recurrent laryngeal nerve injury. The amount of blood loss was lower, lesser swallowing difficulties and of course superior cosmetic results, patient satisfaction and thus better quality of life. However, robotic surgery is associated with a longer operative time, a higher number of excised lymph nodes (40-43).
Despite all these advantages, robotic thyroid surgery is hitting a major obstacle, the high cost of the machine and the instruments. Another barrier is the absence of haptic feedback, this issue might be fixed in the future due to research and investment in this specific subject (44,45).
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Aïdan: Proctor at Intuitive Surgical, Inc. Dr. Bechara has no conflicts of interest to declare.
References
- Gagner M. Endoscopic subtotal parathyroidectomy in patients with primary hyperparathyroidism. Br J Surg 1996;83:875. [Crossref] [PubMed]
- Hüscher CS, Chiodini S, Napolitano C, et al. Endoscopic right thyroid lobectomy. Surg Endosc 1997;11:877. [Crossref] [PubMed]
- Wong KP, Lang BH. Endoscopic Thyroidectomy: A Literature Review and Update. Curr Surg Rep 2013;1:7-15. [Crossref]
- Miccoli P, Berti P, Conte M, et al. Minimally invasive surgery for thyroid small nodules: preliminary report. J Endocrinol Invest 1999;22:849-51. [Crossref] [PubMed]
- Bellantone R, Lombardi CP, Bossola M, et al. Video-assisted vs conventional thyroid lobectomy: a randomized trial. Arch Surg 2002;137:301-4. [Crossref] [PubMed]
- El-Labban GM. Minimally invasive video-assisted thyroidectomy versus conventional thyroidectomy: a single-blinded, randomized controlled clinical trial. J Minim Access Surg 2009;5:97-102. [Crossref] [PubMed]
- Shimizu K, Akira S, Tanaka S. Video-assisted neck surgery: endoscopic resection of benign thyroid tumor aiming at scarless surgery on the neck. J Surg Oncol 1998;69:178-80. [Crossref] [PubMed]
- Ohgami M, Ishii S, Arisawa Y, et al. Scarless endoscopic thyroidectomy: breast approach for better cosmesis. Surg Laparosc Endosc Percutan Tech 2000;10:1-4. [Crossref] [PubMed]
- Ikeda Y, Takami H, Sasaki Y, et al. Endoscopic neck surgery by the axillary approach. J Am Coll Surg 2000;191:336-40. [Crossref] [PubMed]
- Koh YW, Park JH, Kim JW, et al. Endoscopic hemithyroidectomy with prophylactic ipsilateral central neck dissection via an unilateral axillo-breast approach without gas insufflation for unilateral micropapillary thyroid carcinoma: preliminary report. Surg Endosc 2010;24:188-97. [Crossref] [PubMed]
- Lee D, Nam Y, Sung K. Single-incision endoscopic thyroidectomy by the axillary approach. J Laparoendosc Adv Surg Tech A 2010;20:839-42. [Crossref] [PubMed]
- Fan Y, Wu SD, Kong J. Single-port access transaxillary totally endoscopic thyroidectomy: a new approach for minimally invasive thyroid operation. J Laparoendosc Adv Surg Tech A 2011;21:243-7. [Crossref] [PubMed]
- Shimazu K, Shiba E, Tamaki Y, et al. Endoscopic thyroid surgery through the axillo-bilateral- breast approach. Surg Laparosc Endosc Percutan Tech 2003;13:196-201. [Crossref] [PubMed]
- Choe JH, Kim SW, Chung KW, et al. Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surg 2007;31:601-6. [Crossref] [PubMed]
- Duh QY. Presidential address: minimally invasive endocrine surgery—standard of treatment or hype? Surgery 2003;134:849-57. [Crossref] [PubMed]
- Kim SJ, Lee KE, Myong JP, et al. Recovery of sensation in the anterior chest area after bilateral axillo-breast approach endoscopic/robotic thyroidectomy. Surg Laparosc Endosc Percutan Tech 2011;21:366-71. [Crossref] [PubMed]
- Lee KE, Kim HY, Park WS, et al. Postauricular and axillary approach endoscopic neck surgery: a new technique. World J Surg 2009;33:767-72. [Crossref] [PubMed]
- Miccoli P, Bellantone R, Mourad M, et al. Minimally invasive video-assisted thyroidectomy: multi institutional experience. World J Surg 2002;26:972-5. [Crossref] [PubMed]
- Kang SW, Jeong JJ, Yun JS, et al. Robot-assisted endoscopic surgery for thyroid cancer: experience with the first 100 patients. Surg Endosc 2009;23:2399-406. [Crossref] [PubMed]
- Available online: http://www.nndb.com/people/951/000113612/
- Kwoh YS, Hou J, Jonckheere EA, et al. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng 1988;35:153-60. [Crossref] [PubMed]
- Available online: http://guppy.mpe.nus.edu.sg/~mpeangh/niakwu/Pics/Puma.jpg
- Patel V. Robot-asisted laparoscopic dismembered pyeloplasty. Urology 2005;66:45-9. [Crossref] [PubMed]
- Available online: http://www.imperial.ac.uk/mechatronics-in-medicine/research/probot/
- Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cement less total hip arthroplasty. Clin Orthop Relat Res 1992.57-66. [PubMed]
- Available online: http://www.robodoc.com/pro_about_history.html
- Unger SW, Unger HM, Bass RT. AESOP robotic arm. Surg Endosc 1994;8:1131. [Crossref] [PubMed]
- Leal Ghezzi T, Campos Corleta O. 30 years of robotic surgery. World J Surg 2016;40:2550-7. [Crossref] [PubMed]
- Satava RM. Surgical robotics: the early chronicles: a personal historical perspective. Surg Laparosc Endosc Percutan Tech 2002;12:6-16. [Crossref] [PubMed]
- Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin N Am 2003;83:1429-44. [Crossref] [PubMed]
- Available online: https://www.intuitivesurgical.com/company/media/images/
- Litynski GS. Highlights in the History of Laparoscopy. Frankfurt, Germany: Barbara Bernert Verlag, 1996:165-8.
- Reynolds W Jr. The first laparoscopic cholecystectomy. JSLS 2001;5:89-94. [PubMed]
- Kim SM, Chun KW, Chang HJ, et al. Reducing neck incision length during thyroid surgery does not improve satisfaction in patients. Eur Arch Otorhinolaryngol 2015;272:2433-8. [Crossref] [PubMed]
- Arora A, Swords C, Garas G, et al. The perception of scar cosmesis following thyroid and parathyroid surgery: A prospective cohort study. Int J Surg 2016;25:38-43. [Crossref] [PubMed]
- Lee S, Kim HY, Lee CR, et al. A prospective comparison of patient body image after robotic thyroidectomy and conventional open thyroidectomy in patients with papillary thyroid carcinoma. Surgery 2014;156:117-25. [Crossref] [PubMed]
- Song CM, Ji YB, Bang HS, et al. Quality of life after robotic thyroidectomy by a gasless unilateral axillary approach. Ann. Surg Oncol 2014;21:4188-94. [Crossref] [PubMed]
- Lee J, Nah KY, Kim RM, et al. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc 2010;24:3186-94. [Crossref] [PubMed]
- Song CM, Yun BR, Ji YB, et al. Long-Term Voice Outcomes After Robotic Thyroidectomy. World J Surg 2016;40:110-6. [Crossref] [PubMed]
- Lang BH, Wong CK, Tsang JS, et al. A systematic review and meta-analysis comparing outcomes between robotic-assisted thyroidectomy and non-robotic endoscopic thyroidectomy. J Surg Res 2014;191:389-98. [Crossref] [PubMed]
- Son SK, Kim JH, Bae JS, et al. Surgical safety and oncologic effectiveness in robotic versus conventional open thyroidectomy in thyroid cancer: a systematic review and meta-analysis. Ann Surg Oncol 2015;22:3022-32. [Crossref] [PubMed]
- Jackson NR, Yao L, Tufano RP, et al. Safety of robotic thyroidectomy approaches: meta-analysis and systematic review. Head and Neck 2014;36:137-43. [Crossref] [PubMed]
- Kandil E, Hammad AY, Walvekar RR, et al. Robotic thyroidectomy versus nonrobotic approaches: a meta-analysis examining surgical outcomes. Surg Innov 2016;23:317-25. [Crossref] [PubMed]
- Hussain A, Malik A, Halim MU, et al. The use of robotics in surgery: a review. Int J Clin Pract 2014;68:1376-82. [Crossref] [PubMed]
- Kuchenbecker KJ, Gewirtz J, McMahan W, et al. VerroTouch: High-frequency acceleration feedback for telerobotic surgery. In Proceedings, EuroHaptics, 2010:189-96.


